
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please help with question 5

Transcribed Image Text:### Chemistry Problem Set
#### Question 4
**a.** Write a balanced chemical equation for the double replacement reaction. Show all physical states. (Remember that your compounds need to be neutral before balancing or else it will not work).
**b.** How many moles of NaOH are needed to react with 3.50g of Aluminum Sulfate?
**c.** If the solution of NaOH is 0.200M, determine how many mL is needed based on your mole amounts in part b.
---
#### Question 5
**5.** Chalk is composed of calcium carbonate. This water-insoluble compound is formed when a solution of calcium chloride is added to a solution of sodium carbonate. What volume (in mL) of 0.550M calcium chloride is required to completely react with 750.0 mL of 0.250M sodium carbonate solution? (You will need to show all steps in this problem.)
Expert Solution
arrow_forward
Step 1
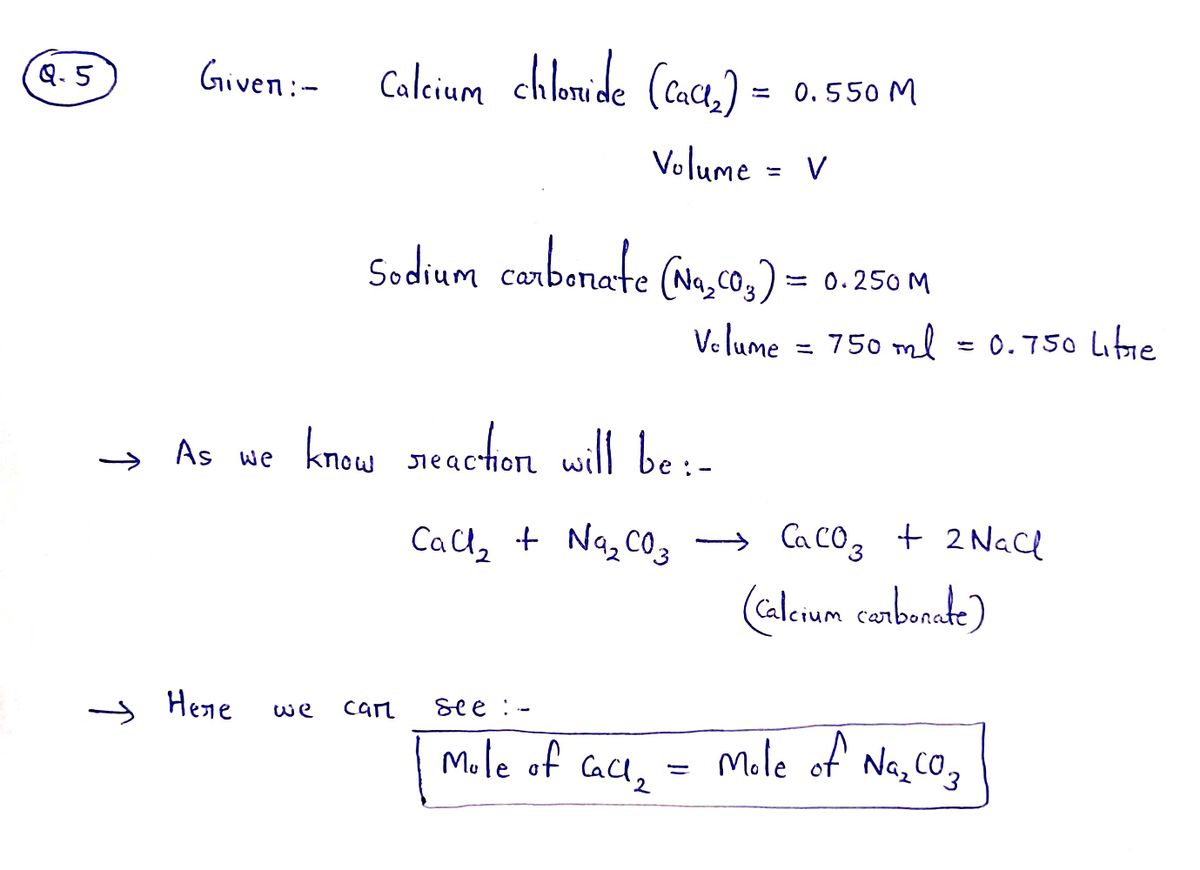
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the value of [N2]eq if [H2]eq = 2.0 M, [NH3]eq = 0.5 M, and Kc = 2. N2(g) + 3H2(g) → 2NH3(g) 0.016 M 0.031 M 0.062 M 0.40 M 62.5 Marrow_forwardIf a sealed, rigid container's pressure is doubled, what will happen to temperature? A) The temperature will decrease by a factor of 2 B) The temperature will decrease by a factor of 4 C) The temperature will increase by a factor of 2 D) The temperature will increase by a factor of 4 E) The temperature will remain the samearrow_forwardWhat is the unit of the answer?arrow_forward
- ml 500 380mL 450 400 350 365mL 370mL None of these 375mL 353mL Line of sightarrow_forwardPlease help me with thesearrow_forwardPlease answer this question as fast as you can please and tahnk you. I will afterwards write an wonderful review on solving the question. Thank you. Which piece of common lab glassware is most likely to be labelled as TC. This label describes its primary use in the lab. Burette Traditional Funnel Volumetric Flask Volumetric Pipettearrow_forward
- Part A If 2.0 x 10-4 moles of S20 in 50 mL solution is consumed in 3 minutes and 17 seconds, what is the rate of consumption of S20 ? Express the rate in mol per liter-second to two significant digits. M 2.128 10 Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining < Return to Assignment Provide Feedbackarrow_forwardKw=1.0x10-¹4 Report all pH values to two places past the decimal. 1. Write the following acid/base reactions. Circle the bases and underline the acids. a. hypochlorous acid (HCIO) reacts with water b. the base ammonia (NH3) reacts with waterarrow_forwardWhich construction would you never use in the laboratory? (A) (B) (C) (D) (E) Construction D Construction B Construction C Construction Earrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY