
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
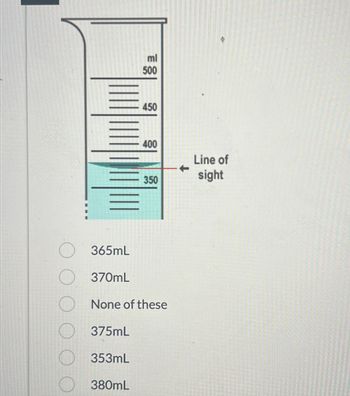
Transcribed Image Text:ml
500
380mL
450
400
350
365mL
370mL
None of these
375mL
353mL
Line of
sight
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the de Broglie relationship to determine the wavelengths of the following objects.arrow_forwardIf l = 3, how many values of ml are possible? -- 1 3 5 7 9arrow_forwardWhat is the volume of liquid in this graduated cylinder? Be sure your answer has a reasonable number of decimal places. normal magnify 120 100. 08, You can click on 60 40 20 mLarrow_forward
- A group of students have a 1000-mL beaker with graduation markings every 50 mL and a volume error of ±1% and they also have a 1000-mL graduated cylinder with graduation markings every 25 mL and a volume error of ±1%. Which piece of equipment can measure 675 mL fairly accurately? Options: neither we would need to purchase a 675-mL piece of glassware if they even make such a thing 1000-mL beaker need more information 1000-mL graduated cylinder botharrow_forward(34.56 cm) * (10.00 cm)*(4.95 cm) = _____________cm3arrow_forwardA bench press bar has a mass of 20.4 kg How many Ib is this? STARTING AMOUNT ADD FACTOR RESET 1000 453.6 0.001 20.4 2.20 45.0 kg Ibarrow_forward
- Height of a 100 mL graduate cylinder in cm 23.42 cm height in m inches km feetarrow_forwardCan you put these lab instruments in order from most accurate to least accurate? (10 mL graduated cylinder, 100 mL graduated cylinder, graduated pipette, fixed volume pipette, and burette)arrow_forwardThis is a picture of a 100-mL graduated cylinder. The value for 'x' says 40 mL and the value for 'y' says a value 10 mL higher than 40. Record the value with proper estimation technique.arrow_forward
- the cubic centimeter (cm3 or cc) has the same volume as a ___. cubic inch centimeter cubic liter millimeterarrow_forwardI am not sure what is being asked for in question #2.arrow_forwardAlie measures out a sample of naphthalene using a graduated cylinder. She reads the measurement as 9.84 mL. The actual volume is 10.29 mL. What is her percent error?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY