
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
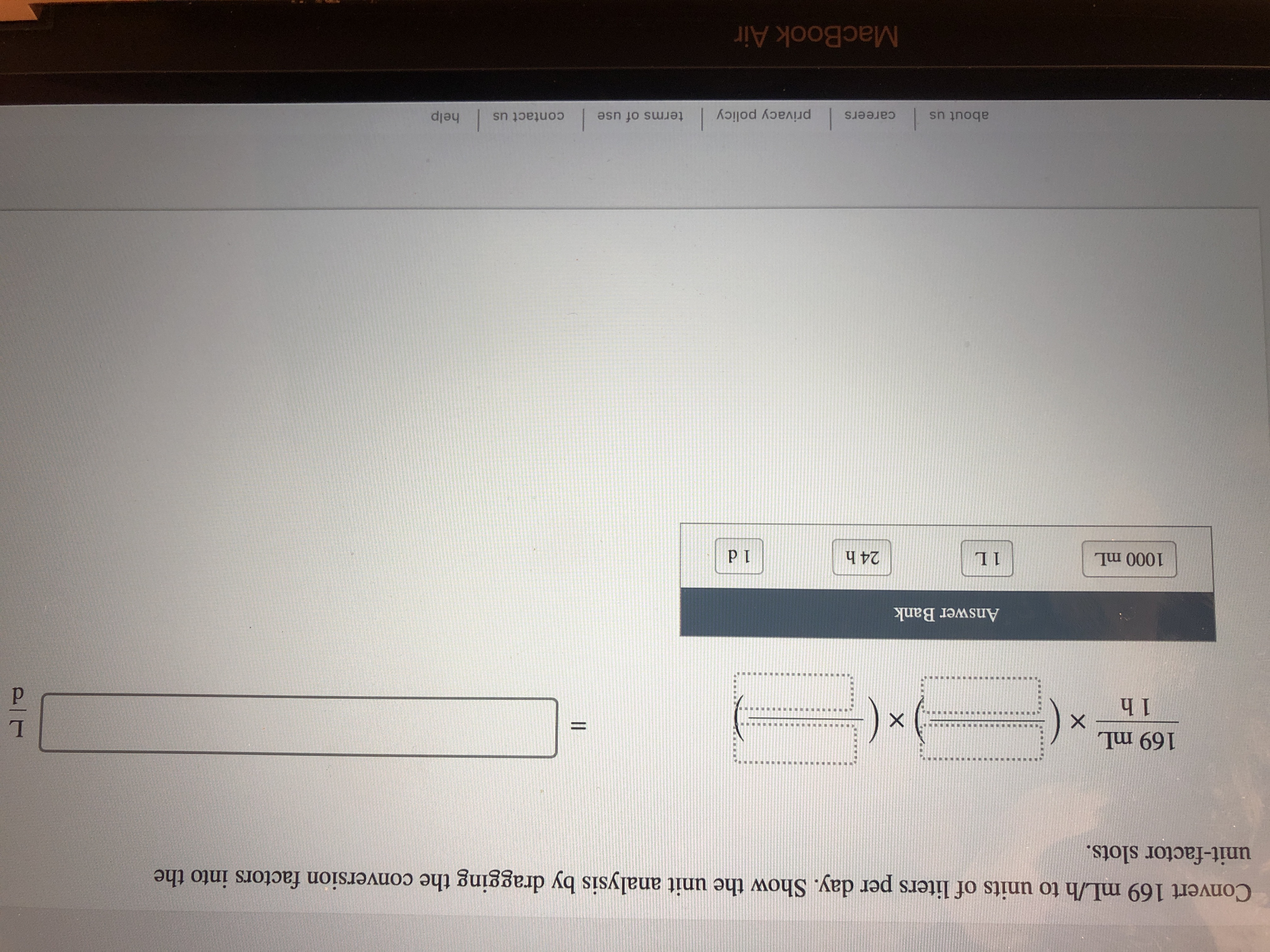
I need help on learning how to solve this type of questions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 0.1 ml 1 ml 1 ml 0.1 ml 1 ml 00000 Original C. D_ 9.9 ml 99 ml 9 ml 9.9 ml 9 ml в D 10 1 ml A 1 ml 1 ml A. 000 B. C. D. Original E. F_ 1 ml 1 ml 1 ml 1 ml 21 22 23 D 1. 32 F 4 A. B. 4, 4. B. 3. 2. 2.arrow_forwardQuestion #2arrow_forwardJust answer the 4th item. The substances are acetone and alcohol.arrow_forward
- I need help on questions 1-4?arrow_forwardThe solvent moves 3 cm in about 10 minutes. Why shouldn't the experiment be stopped at that time instead of waiting 75 minutes for the solvent to move 10 cm? Question 3 options: More time increases the concentration of mixture components. More time allows for better separation of components in a mixture. More time decreases the concentration of mixture components. Instructors don't like students to be finishing their labs too quickly.arrow_forwardthere can only be one answer per question for 4 and 5 you put 2 answers per eacharrow_forward
- Need help with questions 1 and 3. Please see attached image. For question 3, tell me where I need to draw the arrows from and to.arrow_forwardSuppose that in a random selection of 100 colored candies, 25% of them are blue. The candy company claims that thepercentage of blue candies is equal to 24%. Use a 0.05 significance level to test that claim.Identify the null and alternative hypotheses for this test. Choose the correct answer below.arrow_forwardCan you help me solve this? 1ml = 1cm3 = 1arrow_forward
- 6. If the volume of water used in the experiment was 100 mL instead of 50 mL how would the results of the experiment be different? Explain your reasoning.arrow_forwardDetermine mass of cube. Record mass in Lab Data.arrow_forwardRank the boxes above in order from least dense to most dense. Drag the image to place it in the correct order. Please label the boxes in the answer so I know which one you are talking about, please. The boxes are in the images.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY