
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
consider the mechanism of the reaction shown below. Give the structure of the next important organic reaction intermediate alond the reaction coordinate. Your answer could be the final product
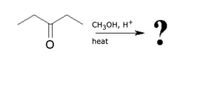
Transcribed Image Text:CH3OH, H+
heat
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Compound A reacts with B in the presence of sodium hydride to give C as the majority product.1. Give the detailed mechanism of the formation of C and draw the product C in the box above.2. Draw the energy diagram of the reaction. Indicate with an arrow the rate-determining step of the reaction then draw its transition state3.The rate of the reaction observed is 5 x 10-2 M.s-1 when the concentrations of A and B are 0.2 M and 0.1 M respectively.Determine the rate of the reaction if the concentrations of A and B are now 0.3 M and 0.2 M respectively. Show your calculationarrow_forwardComplete the following two-step reaction sequence by providing a plausible curved-arrow mechanism for each step of the reaction sequence that leads to the formation of the final products. For the first step, you only need to show the mechanism for the conversion of the first equivalent of the starting alcohol. Be sure to draw structures for all key intermediates and major products and show formal charges and electron flow with curved arrows. Each curved arrow must begin from either a bonding pair or lone pair of electrons and point at an atom or the right place of a bond. Show lone pairs of electron if needed. If one lone pair is shown on an atom, the rest of lone pairs on the same atom must be shown as well.arrow_forwardNonearrow_forward
- Please don't provide handwritten solution ....arrow_forwardPayalbenarrow_forwardThis reaction is also an important reaction of the tricarboxylic acid cycle in cells, wherein the reaction occurs in neutral solution, so the acid groups are both ionized to the carboxylate form. The reaction is catalyzed by the stereospecific enzyme fumarase that utilizes only the trans form of 2-butenedioate ion (also known as fumarate) and produces only the (S)-2-hydroxysuccinate enantiomer (also known as (S)-malate). Draw the correct stereochemical structures of these two compounds of the fumarase-catalyzed reaction. Be sure to include all hydrogen atoms and show the carboxylates as anions.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY