
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
PLEASE SELECT ONLY 1 OPTION FROM THE FOLLOWING ONLY OPTIONS:
- Elimination 2nd order
- Radical Halogenation
- Electrophilic
Aromatic Substitution Alkene Addition- Substitution Nucleophilic 2nd order
- Pericyclic
- Organometallic Coupling React
- Elimination 1st order
- Oxidation
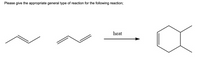
Transcribed Image Text:Please give the appropriate general type of reaction for the following reaction;
heat
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Pick your top 3 reactions from the following in chapter 8: Reaction 1: Addition of H-X to Alkenes Reaction 2: Addition of Water to Alkenes Reaction 5: Addition of Alcohols to Alkenes Reaction 6: Addition of X-X to Alkenes Reaction 7: Addition of X-X to Alkenes in a Polar Protic Solvent Reaction 8: Epoxidation of Alkenes QUESTION: Show the mechanism and formation of all products for your alkene for the selected reaction As part of your submission you must: An explanation of each step of your reaction (as if you were teaching someone) Identify the major product Explanation for why you selected the major product Please Give a detailed EXPLANATION for the reactionarrow_forwardIn an E2 mediated elimination reaction of an alkyl halide (H-X) using NaOCH3 as base, which of the following statement is not true? O The reactivity order for alkyl halides (RX) is tertiary secondary> primary. O The rate of reaction = k2 (alkyl halide] [NaOCH₂] O The reaction occurs in a single step. O The rate is independent of the leaving group.arrow_forwardIdentify the electrophile in the following electrophilic addition reaction step. Explain your choice.arrow_forward
- 29 minutes, 42 seconds. Question Completion Status: A Moving to another question will save this response. Question 15 What is not an expected product of the following allylic substitution reaction? NBS, hv Br Br Compounds II and II Compound II only O Compound I only O Compound II only A Moving to another question will save this response O O Carrow_forwardWhich statement(s) is(are) true concerning the rate-limiting step involving the benzene ring? CI AICI3 O aromaticity is restored aromaticity is disrupted proton transfer occurs carbocation intermediate forms O the benzene ring functions as the nucleophile the benzene ring functions as the electrophilearrow_forwardWhich one of the following molecules CANNOT undergo a nucleophilic aromatic substitution reaction? o O2N. O,N `NO2 NO2 F NO2 NO2 NO2 NO2 `NO2 NO2 Brarrow_forward
- A common alkene starting material is shown below. Predict the major product for each reaction. Ignore any inorganic byproducts.arrow_forwardThe following reaction is electrophilic substitution: 3- [Pt(CH₂) (N) [Pt(CH3)(CI) (PMePh₂)₂] + N° (PMePh₂)₂] + CI Select one: O True O Falsearrow_forwardIt's very difficult to see where arrows goarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY