
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
A vicinal
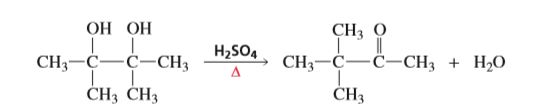
Transcribed Image Text:CH3 O
CH3-C-C-CH3
НО НО
CH3-C-C-CH3
H2SO4
Н-О
CHз CH3
ČH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- According to Le Chatelier's Principle, acetal product formation tends to INCREASE (rather than decrease) when more alcohol is added to the reaction mixture. True or False?arrow_forwardDraw the major organic product for the reaction of this compound with PBr3.arrow_forwardSorbitol is a sweetener often used in chewing gum and diet drinks. It is obtained by the following reaction. Which of the following functional group transformations occur during this reaction? СНО CH2OH Н-с-ОН Н-с-ОН Но-с-н Н2 Но-с-н Н-с-ОН Н-с-оН Н-с-ОН н-с-он CH2OH CH-Он glucose sorbitol a. The aldehyde becomes an alcohol. b. An alcohol becomes an aldehyde. c. An alcohol disappears. d. A new aldehyde appears. e. All of the abovearrow_forward
- When butanone reacts with ethylene glycol and an acid catalyst it forms an ketal that will react with only one of the following. Which one? A) NaOH / H2O B) HCI/H,0 C) LiAIH D) CHIMgBr E) NaCNarrow_forwardWhich of the following reagents would lead to a product that has two functional groups: an alcohol and an ether? NaOH/H₂O H3O+/H₂O HC1 CH3CH₂OH/CH3CH₂O™¹Na H₂C CH₂arrow_forwardReaction of 1-butanol with periodane gives Butanal Propanoic acid 2-Butanone Butanoic acid as the major product.arrow_forward
- A dialkyl-substituted benzene, C14H22, is treated with basic potassium permanganate, followed by acid workup. The same dialkyl-substituted benzene was recovered afterward from the reaction mixture. Draw the structure of the compound.arrow_forwardIn the following reaction, a primary alcohol is fully oxidized to a carboxylic acid. Draw the aldehyde intermediate that was formed.arrow_forwardDraw the structure(s) of the major organic product(s) of the following reaction. Draw a structural formula for the enol form of the carbonyl compound below.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY