
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
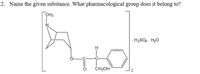
Transcribed Image Text:2. Name the given substance. What pharmacological group does it belong to?
CH3
N
. H2SO4 . H20
CH2OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. What What is the ooter of a reaction which has the kade law equalio , hate =KLAJ B]"?arrow_forwardHigh fructose corn syrup (HFCS) is a sweetener that was designed in the 1970s, which takes corn starch and chemically modifies it to have a mixture of the constitutional isomers glucose and fructose (both of the formula C6H12O6). HFCS 55 is the type found in US sodas (like Coca Cola) and it contains 55.1% fructose molecules and 44.9% glucose molecules. Given that information, calculate how many molecules of fructose would be found in a can of coca cola if the amount of HFCS used were 67.5 g? Use the correct number of significant figures. *Input answer as: 6.022x10^23arrow_forward1. What is the mass percent of potassium chloride in a solution that is made by dissolving 15.0 g KCI in 100.0 g H,O? Show work. Parrow_forward
- Determine the mass of the water in the sample based on the following data to teo decimal places. Mass of crucible and cover- 50.02 Mass of crucible, cover and sample before heating-51.04 Mass of crucible, cover and sample after heating-50.86arrow_forwardA solution is prepared by dissolving 23.4 g of CaCl2 (MW = 110.98 g/mol) in 355 mL of water. The density of the resulting solution is 1.05 g/mL. The concentration of CaCl2 in this solution is ________ molar.arrow_forward13. Nitric acid, HNO3, is a strong acid in aqueous solutions. It is sold commercially as a concentrated solution with a density of 2.14 kg.L-¹ and containing 58% mass of HNO3. We introduce 10.0 mL of this solution in a 250.00 mL volumetric flask which is then filled to the mark with distilled water. This solution is called S. S is then diluted 100 times to afford solution S'. a. What is the definition of a strong acid? | b. What is the concentration of solution S"? c. What is the pH of solution S'?arrow_forward
- 1. CH3CH₂COCI, AICI3 2. Br₂, CH3CO₂H 3. K* tBuO™ 4. OsO4 then NaHSO3 5. H₂CRO4arrow_forward(i) NBS, MeOH ? (ii) Na OEt, ELOH OMe O A. ÓEt OEt O B. ÔMe Br OC. OMe OD. Br OMearrow_forwardMass of crucible and cover 12.73g 2. Mass of crucible, cover, and sample 24.11g 3. Mass of original sample 24.11- 12.73g= 11.97g 4. Mass of crucible, cover, and sample after 1st heating 20.01g 6. Mass of crucible, cover, and sample after 2nd heating (assume that this step was done) 19.98 g 7. Total mass lost by sample during heating what is the percentage of water in the sample? and what is the total mass lost by the sample?arrow_forward
- The excess reactant will always have the greater volume of the reactants. True/Falsearrow_forwardity College-CHM 101 (Section 90) - Spring20 - MARX > Activities and Due Dates > Ch 7 HW O Assignment Score: O Resources Ly Give Up? 50.1% O Hint Check Answer < Question 16 of 28 The combustion of ethane (C,H.) produces carbon dioxide and steam. 2 C,H,(s) +70,(g) → 4 CO,(g) + 6 H, O(g) How many moles of CO, are produced when 5.05 mol of ethane is burned in an excess of oxygen? moles of CO,: mol terms of use contactus help about us carecrs privacy policy MacBook Aarrow_forward5) If all the NaHCO3 in bag 2 reacted, calculate the number of moles of gas produced? tates) E Focusarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY