
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
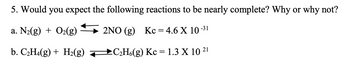
Transcribed Image Text:5. Would you expect the following reactions to be nearly complete? Why or why not?
a. N₂(g) + O₂(g)
2NO (g) Kc = 4.6 X 10-³1
b. C₂H4(g) + H₂(g)
→C₂H6(g) Kc = 1.3 X 10 21
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 16. Give the product side of the balanced chemical reaction for the following reaction: C6H12 (1) + O2 (g) → Select one: a.none of these b. 6 CO2 (g) + 3 H20 (g) c. CO2 (g) + H20 (g) d. 6 CO2 (g) + 6 H20 (g) e. 12 CO2 (g) + 12 H20 (g) f. 12 CO2 (g) + 6 H20 (g) 17 Giyen the reaction below, when 23.70 moles manganese acetate react, harrow_forward10. Determine the mass percent composition of each element in calcium silicate CaSiO3. 11. A compound is found to contain 23.3% magnesium, 30.7% sulfur, and 46.0% oxygen. What is the empirical formula of this compound? 12. The complete combustion of acetylene (C₂H₂) is described by the following reaction: CHz + O2 → CO2 + H2O A. Balance the above equation with the lowest set of whole numbers. B. If 2.35g C₂H₂ is burned with enough oxygen, how many grams of CO₂ will be formed?arrow_forwardWhich statement about the following two reactions is correct? Reaction I: A → 2C +B Ea = 54 kJ/mol %3D Reaction II: D→ E + F Ea = 132 kJ/mol %3D O Not enough information provided to compare k. O k1 = k2 %3D O k1 k2arrow_forward
- The molecular formula and the empirical formula for this hydrocarbon are a. C5 H10 and CH2 b. C5 H10 and CH3 c. C4H8 and CH2 d. C4H8 and CH3arrow_forwardPLEASE SHOW YOUR WORK (THIS IS NOT A GRADED ASSIGNMENT)arrow_forward7. Consider the reaction: 2 ZnSe +3 02 )2 Zno s +2 SO,a Which value is closest to the mass of Zno a produced when 50.0 g ZnS is heated in an open vessel until no further weight loss is observed? (molar masses O, [32 g), So, [64 g), Zno [81 g], ZnS [97 g]) a. 25 g b. 40 g c. 50 g d. 60 g (s)arrow_forward
- T Ma M PA USN Be * Ma E Ad G ph ph Do E Un O Ac. 6 Pec O Na O Pe Со Bas 3 Pe Stu "app.101edu.co Question 11 of 11 What is the mass in grams of H2 that can be formed from 55.8 grams of NH3 in the following reaction? 2 NH:(g) → 3 H:(g) + N:(g)arrow_forwardJj.70.arrow_forward00 %24 4+ dl w/ S' Enter your answer in the provided box. Using the balanced equation for the combustion of ethanol, answer the following question. C2H,O(1) + 3 02(g) – 2 CO2(g) + 3 H,0 (g) ethanol How many grams of H,0 are formed from 1.2 mol of ethanol? OH 8 #3 5. 9. 2 4 0Oarrow_forward
- Write a balanced chemical equation based on the following description: solid potassium oxide reacts with liquid water to produce aqueous potassium hydroxidearrow_forwardHow many grams of oxygen gas (O2) is required for the complete combustion of 25.5 g of benzene (C6H6)? 2C6H6(1) + 1502(g) 12 CO2(g) + 6H2O(g)arrow_forward2 NaHCO3 (s) → N22CO3 (s) + CO2 (g) + H2O (g) 1. Calculate the moles of NaHCO3 in 3.00 g of NaHCO3. 3,00 84 2. Using your answer to question one, if 1 mole of Na2CO3 is produced for every 2 moles of NaHCO3, how many moles of Na2CO3 would theoretically be produced in the reaction? 3. How many grams of Na2CO3 would theoretically be produced in the reaction? 4. If the actual mass of Na2CO3 produced is 1.25 g, what is the percent yield of Na2CO3? Actual yield (g) Theoretical yield (g) Percent yield = 100% 5. Calculate the theoretical mass of H2O produced from the decomposition of 3.00 g NaHCO3 using similar calculations as above.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY