
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
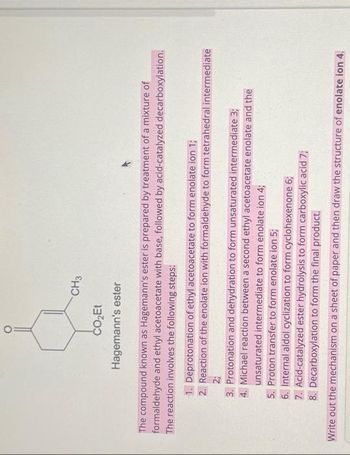
Transcribed Image Text:CH3
CO₂Et
Hagemann's ester
The compound known as Hagemann's ester is prepared by treatment of a mixture of
formaldehyde and ethyl acetoacetate with base, followed by acid-catalyzed decarboxylation.
The reaction involves the following steps:
1. Deprotonation of ethyl acetoacetate to form enolate ion 1;
2. Reaction of the enolate ion with formaldehyde to form tetrahedral intermediate
2;
3. Protonation and dehydration to form unsaturated intermediate 3;
4. Michael reaction between a second ethyl acetoacetate enolate and the
unsaturated intermediate to form enolate ion 4;
5. Proton transfer to form enolate ion 5;
6. Internal aldol cyclization to form cyclohexenone 6;
7. Acid-catalyzed ester hydrolysis to form carboxylic acid 7;
8. Decarboxylation to form the final product.
Write out the mechanism on a sheet of paper and then draw the structure of enolate ion 4.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- May you please help me with this ochem question?arrow_forwardRank the compounds in order of electrophilicity. (X > Y means X is more electrophilic than Y). Acyl chlorides > Anhydrides > Amides > Aldehydes Amides > Acyl chlorides > Anhydrides > Aldehydes Aldehydes > Acyl chlorides > Anhydrides > Amides O Acyl chlorides > Anhydrides > Aldehydes > Amidesarrow_forward1) How might you convert geraniol into either ethyl geranylacetate or geranylacetone? Geraniol CH₂OH CO₂Et Ethyl geranylacetate the Geranylacetonearrow_forward
- Quinapril (trade name Accupril) is used to treat high blood pressure andcongestive heart failure. One step in the synthesis of quinapril involvesreaction of the racemic alkyl bromide A with a single enantiomer of theamino ester B. Given the structure of quinapril, which one of these two products isneeded to synthesize the drug?arrow_forwardTreatment of ethyl acetoacetate with NaOEt (2 equiv) and BrCH2CH2Br forms compound X. This reaction is the first step in the synthesis of illudin-S, an antitumor substance isolated from the jack-o'-lantern, a poisonous, saffron-colored mushroom. What is the structure of X?arrow_forwardProstaglandins are a class of eicosanoids, fatty acid derivatives involved in a variety of important phenomena, including fever, inflammation, and pain. The first step in prostaglandin synthesis involves the conversion of the 20-carbon fatty acid arachidonic acid to PGG2 by the enzyme prostaglandin endoperoxide synthase, or cyclooxy: Ibuprofen is one of several inhibitors of cyclooxogenase used therapeutically. Arachidonic acid 20₂ 0.5 1.0 1.5 2.5 3.5 cyclooxrygenase PGG₂ JOH محمد The following kinetic data were obtained for the enzyme cyclooxygenase in the absence of any inhibitor (1), and in the presence of ibuprofen (2) at a concentration of 48 [arachidonic acid] (uM) (1) v(uM/min) (2) v(uM/min) 23.5 32.2 36.9 41.8 44.0 16.67 25.25 30.49 37.04 38.91 Ibuprofen A. Plot the data in standard Michaelis-Menten form as well as in double-reciprocal form. B. Determine Vmax and Km for the enzyme. C. Based on this limited data set, what type of inhibition does ibuprofen likely exhibit?…arrow_forward
- From each pair of compounds, choose the one with the indicated property and explain your choice. a) is racemized when placed in basic solution: (R)-4-methyl-2-hexanone or (R)-3-methyl-2-hexanone b) forms an enamine when treated with a ketone: diethylmethylamine or diethylaminearrow_forwardWhat is the product formed by hydrolysis of the following lactone with acid? А. ос B. H3O+ ОН с. OH D. будося OH ОНarrow_forwardAziridines are heterocycles that contain an N atom in a three-membered ring. Like epoxides, aziridines are strained and reactive because the 60° bond angles of the three-membered ring deviate greatly from the theoretical tetrahedral bond angle. One step in the synthesis of the drug oseltamivir (trade name Tamiflu, Section 3.2) involves the conversion of amine X to diamine Y, a reaction that occurs by way of an intermediate aziridine. Draw a stepwise mechanism for the conversion of X to Y. Indicate the structure of the aziridine intermediate, and explain the trans stereochemistry of the two amines in Y.arrow_forward
- Draw the carbonyl and amine productarrow_forwardA step in a synthesis of PGE1 (prostanglandin E1, alprostadil) is reaction of a trisubstituted cyclohexene with bromine to form a bromolactone. Alprostadil is used as temporary therapy for infants born with congenital heart defects that restrict pulmonary blood flow. It brings about dilation of the ductus arteriosus, which in turn increases blood flow in the lungs and blood oxygenation. Propose a mechanism for formation of This bromolactone, and account for the observed stereochemistry of each substituent on the cyclohexane ring.arrow_forwardRank the following compounds in order of increasing basicity and explain the order you chose.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY