
Pushing Electrons
4th Edition
ISBN: 9781133951889
Author: Weeks, Daniel P.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
(Time constraint) Please provide the IUPAC name
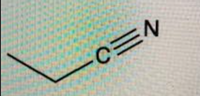
Transcribed Image Text:CEN
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Describe how the following changes are brought about: (i) Pig iron into steel. (ii) Zinc oxide into metallic zinc. (iii) Impure titanium into pure titanium.arrow_forwardA chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of carbon tetrachloride, diethylamine, pentane, acetone, and tetrahydrofuran. The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from his collection of Material Safety Data Sheets (MSDS), the chemist finds the following information: liquid density - 3 1.6 g·cm carbon tetrachloride diethylamine - 3 0.71 g·cm pentane 3 0.63 g cm 3 0.79 g cm acetone tetrahydrofuran - 3 0.89 g cm Next, the chemist measures the volume of the unknown liquid as 1.427 L and the mass of the unknown liquid as 894. g. Calculate the density of the liquid. Round 3 your answer to 3 significant digits. |lg.cm Given the data above, is it possible to identify the liquid? yes no carbon tetrachloride diethylamine If it is possible to identify the liquid, do so. pentane acetone tetrahydrofuranarrow_forward(i) H3C CI (ii) HO OHarrow_forward
- Which branch of chemistry would be the most relevant to a learner that plans on becoming a nutritionist? A physical chemistry B C D biochemistry inorganic chemistry nuclear chemistryarrow_forwardWhich of the following alkenes is the most stable? O O The sauna premiuarrow_forwardA chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of carbon tetrachloride, diethylamine, methyl acetate, tetrahydrofuran, and acetone. The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from his collection of Material Safety Data Sheets (MSDS), the chemist finds the following information:arrow_forward
- Class Participation Q#5 CH3 Ĉ -CI Ho cholesterol oleic acid,arrow_forwardCalculate the cost per individual molecule of your pain reliever. Acetaminophen has the chemical formula C8H9NO2. Ibuprofen has the chemical formula C13H18O2. Paracetamol has the chemical formula C8H9NO2.arrow_forwardA chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of diethylamine, pentane, acetone, carbon tetrachloride, and tetrahydrofuran. The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from her collection of Material Safety Data Sheets (MSDS), the chemist finds the following information: liquid density g 0.71 mL diethylamine olo Ar pentane 0.63 mL g 0.79 mL acetone g 1.6 mL carbon tetrachloride g 0.89 mL tetrahydrofuranarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning



Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning