
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Can someone explain how to convert a condensed formula to a bond. I get confused about atoms that aren’t C or H and when to branch . Thanks for any explanation.
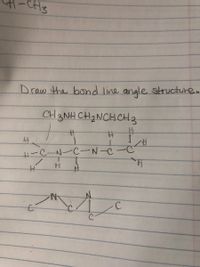
Transcribed Image Text:CH3
Draw the bond line angle structure..
CH 3NH CH2NCHCH3
%23
甘
H.
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which atomic orbitals overlap to form the bonds in ClF?arrow_forwardExplain in terms of bonding theory why all four hydrogen atoms of allene, H2CCCH2, cannot lie in the same plane.arrow_forwardIn each of the following molecules, a central atom is surrounded by a total of three atoms or unshared electron pairs: SnCl2, BCl3, SO2. In which of these molecules would you expect the bond angle to be less than 120? Explain your reasoning.arrow_forward
- Consider the following flat drawing of methane (CH4) . a. What is HCH bond angle implied by this drawing if you assume it is flat? b. Are the electron domains of this flat CH4 spread out as much as possible? c. Use model materials to make a model of CH4 (methane). If you assembled it correctly, thefour bonds (bonding electron domains) of your model will be 109.5° apart. d. In which representation, the drawing above or the model in your hand (circle one) are theH’s of CH4 more spread out around the central carbon? e. Confirm that your model looks like the following drawing. The wedgebond represents a bond coming out of the page, and the dash bondrepresents a bond going into the page f. You will often see methane drawn as if it were flat (like on the previous page). Why is thismisleading, and what is left to the viewer’s imagination when looking at such a drawing?arrow_forwardA compound of chlorine and fluorine, CIFx, reacts at about 75C with uranium to produce uranium hexafluoride and chlorine fluoride, CIF. A certain amount of uranium produced 5.63 g of uranium hexafluoride and 457 mL of chlorine fluoride at 75C and 3.00 atm. What is x? Describe the geometry, polarity, and bond angles of the compound and the hybridization of chlorine. How many sigma and pi bonds are there?arrow_forwardWhich of the following statements is/are true? Correct the false statements. a. The molecules SeS3. SeS2, PCl5, TeCl4, ICl3, and XeCl2 all exhibit at least one bond angle which is approximately 120. b. The bond angle in SO2 should be similar to the bond angle in CS2 or SCI2. c. Of the compounds CF4, KrF4, and SeF4, only SeF4 exhibits an overall dipole moment (is polar). d. Central atoms in a molecule adopt a geometry of the bonded atoms and lone pairs about the central atom in order to maximize electron repulsions.arrow_forward
- What are the bond angles predicted by the VSEPR model about the carbon atom in the formate ion, HCO2? Considering that the bonds to this atom are not identical, would you expect the experimental values to agree precisely with the VSEPR values? How might they differ?arrow_forwardUse Figs. 4-54 and 4-55 to answer the following questions. a. Would the bonding molecular orbital in HF place greater electron density near the H or the F atom? Why? b. Would the bonding molecular orbital have greater fluorine 2p character, greater hydrogen 1s character, or an equal contribution from both? Why? c. Answer the previous two questions for the antibonding molecular orbital in HF.arrow_forwardThe ions ClF2 and ClF2+ have both been observed. Use the VSEPR model to predict the FClF bond angle in each.arrow_forward
- Compare Figs. 4-47 and 4-49. Why are they different? Because B2 is known to be paramagnetic, the 2p and 2p molecular orbitals must be switched from the first prediction. What is the rationale for this? Why might one expect the 2p to be lower in energy than the 2p? Why cant we use diatomic oxygen to help us decide whether the 2p, or 2p, is lower in energy?arrow_forwardNonearrow_forwardWhat atomic or hybrid orbitals make up the sigma bond between C2 and H in ethane, CH3CH3 ?(C2 is the second carbon in the structure as written.) orbital on C2 + orbital on H What is the approximate C1-C2-H bond angle ? ... fill in the blank 3 °arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning