
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
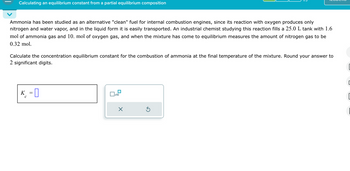
Transcribed Image Text:Calculating an equilibrium constant from a partial equilibrium composition
Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only
nitrogen and water vapor, and in the liquid form it is easily transported. An industrial chemist studying this reaction fills a 25.0 L tank with 1.6
mol of ammonia gas and 10. mol of oxygen gas, and when the mixture has come to equilibrium measures the amount of nitrogen gas to be
0.32 mol.
Calculate the concentration equilibrium constant for the combustion of ammonia at the final temperature of the mixture. Round your answer to
2 significant digits.
K
с
=
☐ x10
☑
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Coal can be used to generate hydrogen gas (a potential fuel) by the following endothemic reaction (heat is on the reactant side of the equation). C (3) + H20 (g) = co (g) + H2 (g) If this reaction mixture is at equilibrium, predict whether each of the following will resuit in the formation of additional hydrogen gas, the formation of less hydrogen gas, or have no effect on the quantity of hydrogen gas. Part A adding more C to the reaction mixture the formation of additional hydrogen gas the formation of less hydrogen gas O no effect on the quantity of hydrogen gasarrow_forwardSteam reforming of methane ( CH, ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which Is the starting point for many 4 Important Industrial chemical syntheses. An Industrial chemist studying this reaction fills a 50.0 L tank with 3.7 mol of methane gas and 4.9 mol of water vapor, and when the mixture has come to equilibrium measures the amount of hydrogen gas to be 7.8 mol. Calculate the concentration equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits. K =] x10 Check Explanation 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy Accessib 6. M9 hparrow_forwardA student adds 0.600 mol of methane gas, CH4(g) into a 2.00 L container and the methane forms ethyne, C2H2(g) and hydrogen gas, H2(g). At equilibrium, the container was found to contain 0.045 mol/L C2H2.a) Calculate the initial concentration of CH4. Then complete an ICE Table to organize the initial, change and equilibrium concentrations for this equilibrium system. Show any calculations needed. 2CH4(g) ⇄ C2H2(g) + 3H2(g)b) Calculate the equilibrium constant, Keq, for this equilibrium. Show all work.c) What is the equilibrium concentration of H2(g) ?arrow_forward
- Calculating an equilibrium constant from a partial equilibrium compositionarrow_forwardSteam reforming of methane ( CH, ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 25.0 L tank with 1.5 mol of methane gas and 2.0 mol of water vapor, and when the mixture has come to equilibrium measures the amount of hydrogen gas to be 2.7 mol. Calculate the concentration equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits. K. = Iarrow_forwardIf an equilibrium system has a high value (>1) for K, what does this indicate? How will the concentration of reactants compare to the concentration of products? [P] [R]arrow_forward
- For which test tube should your value for the equilibrium constant be most reliable? Justify your selection based on your data and your laboratory experience in completing this procedure. Equilibrium Constant, K Test Tube 1 - 75.0 Test Tube 2 - 88.2 Test Tube 3 - 167 Test Tube 4 - 161 Test Tube 5 - -598 Average Kc = -21.4arrow_forwardWhat does it mean for a chemical reaction to reach equilibrium? The rates of the forward and reverse reactions are equal. The concentrations of the reactants and products are also equal. The rates of the forward and reverse reactions are increasing. The concentrations of the reactants and products are equal. The rates of the forward and reverse reactions are equal. The concentrations of the reactants and products are constant. The rates of the forward and reverse reactions are increasing. The concentrations of the reactants and products are also increasing.arrow_forwardCalculate the equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture.arrow_forward
- Calculating an equilibrium constant from a partial equilibrium.. Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a 2.0 L flask with 3.9 atm of sulfur dioxide gas and 0.74 atm of oxygen gas, and when the mixture has come to equilibrium measures the partial pressure of sulfur trioxide gas to be 1.2 atm. Calculate the pressure equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to 2 significant digits. K, = [ %3D do x10 Ar Eplanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility FEB tv 23 30 MacBook Air DII DD F12 F11 F10 F9 80 888 F8 F7 F5 F6 F4 F3 esc F2 F1 $ % @ 8. 3 4 5 6 2 Uarrow_forwardCalculating an equilibrium constant from a partial equilibrium composition Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only nitrogen and water vapor, and in the liquid form it is easily transported. An industrial chemist studying this reaction fills a 2.0 L flask with 1.2 atm of ammonia gas and 3.7 atm of oxygen gas, and when the mixture has come to equilibrium measures the partial pressure of water vapor to be 1.3 atm. Calculate the pressure equilibrium constant for the combustion of ammonia at the final temperature of the mixture. Round your answer to 2 significant digits. K = [] P x10 X ? olo 18 Ar 8.arrow_forwardA chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: N₂(g) + 2 H₂O(g) 2 H₂(g) + 2NO(g) = -9 K₂=3.x 107 He fills a reaction vessel at this temperature with 15. atm of nitrogen gas and 7.8 atm of water vapor. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of NO, using only the tools available to you within ALEKS? If you said yes, then enter the equilibrium pressure of NO at right. Round your answer to 1 significant digit. O yes O no 0 atm 0 10 Xarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY