
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Please do not rely too much on chatgpt, because its answer may be wrong. Please consider it carefully and give your own answer. You can borrow ideas from gpt, but please do not believe its answer.Very very grateful!Please do not rely too much on chatgpt, because its answer may be wrong. Please consider it carefully and give your own answer. You can borrow ideas from gpt, but please do not believe its answer.
and and Very very grateful!
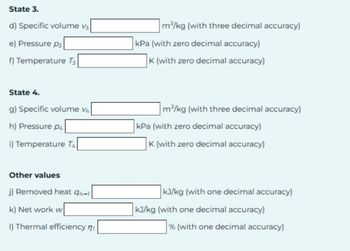
Transcribed Image Text:State 3.
d) Specific volume v3
e) Pressure p
f) Temperature T3
m³/kg (with three decimal accuracy)
kPa (with zero decimal accuracy)
K (with zero decimal accuracy)
State 4.
g) Specific volume v4
h) Pressure P4
i) Temperature T4
m³/kg (with three decimal accuracy)
kPa (with zero decimal accuracy)
K (with zero decimal accuracy)
Other values
j) Removed heat 94-1
k) Net work w
I) Thermal efficiency n
kJ/kg (with one decimal accuracy)
kJ/kg (with one decimal accuracy)
% (with one decimal accuracy)
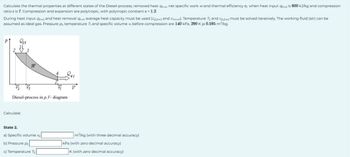
Transcribed Image Text:Calculate the thermal properties at different states of the Diesel-process, removed heat 94-1, net specific work wand thermal efficiency n when heat input 2-3 is 600 kJ/kg and compression
ratio & is 7. Compression and expansion are polytropic, with polytropic constant k = 1.2.
During heat input 9243 and heat removal 94-1 average heat capacity must be used (Cp2-3 and CV,4-1). Temperature T3 and Cp,243 must be solved iteratively. The working fluid (air) can be
assumed as ideal gas. Pressure pi, temperature T₁ and specific volume v₁ before compression are 140 kPa, 290 K ja 0.595 m³/kg.
W
V₂ V3
Diesel-process in p, V- diagram
Calculate:
State 2.
a) Specific volume v₂
m³/kg (with three decimal accuracy)
b) Pressure P2
c) Temperature T₂
kPa (with zero decimal accuracy)
K (with zero decimal accuracy)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- Find the air-fuel ratio of a four-stroke, single-cylinder, air-cooled engine with fuel consumptionatime for 10 cc is 20.4 s and ay ansumption time for 0.1 m3 is IS 3 Find also brabre bultfuel consumption in g/kW 4.3 3 brake thermal efficiency. Assıunhe the density of air as 1.175 kg/m³ &ck specific gravity of fuel to be 0.7. The lower heating value of fuel is 43 MJ/kg and the dynamometer constant is 5000. (imm "he load is 17 kg at the of 3000 rpm.arrow_forwardplease solve for Darrow_forwardThe motor of an electrical appliance consumes 18 000 J. during itsoperation, 9450 J dissipated in heat, 250 J were used forproduce light and 350 J are required to power the electronic panel ofdevice. What is the fuel efficiency of the engine of this device ?arrow_forward
- Dont ignore solve for me All ok take ur timearrow_forwardOpen with Google Docs 4-43 Air expands through a turbine from 10 bar, 900 K to 1 bar 500 K. The inlet velocity is small compared to the exit veloc- ity of 100 m/s The turbine operates at steady state and devel- ops a power output of 3200EW Heat transfer between the tur- bine and its surroundings and potential energy effects are negligible. Calculate the mass tlow rate of air, in kg/s, and the exit area, in m. 499 Relrarrow_forwardPls. Answer... A steam engine boiler is maintained at 250°C and water is converted into steam. This steam is used to do work and heat is ejected to the surrounding air at temperature 300K. Calculate the maximum efficiency it can have in percent?arrow_forward
- Solve it correctly please. I will rate accordinglyarrow_forwardProvide the diagram, given, and ste by step solution. An air standard engine has a compression ratio of 15 and a cut off ratio of 3. If theintake air pressure and temperature are 100 kpa and 28 degree Celcisus, find the work in KJ per kg.arrow_forwardA computer lab filled with 20 computers and 15 students is cooled by an air conditioningunit on a day where it is 90 oF outside. The room temperature is 72 oF. Assume an averagestudent generate 90 W of heat and each computer generates 150 W of heat. If the COP ofthe air conditioner is 2.5, what power input is required, in kW? If the air conditioneroperated at its maximum coefficient of performance, what power input would be required?arrow_forward
- A test on a single cylinder, four stroke oil engine having a bore of 15cm and stroke 30 cm gave the following results: speed 300 rpm, brake torque 200 Nm, indicated mean effective pressure 7 bar, fuel consumption 2.4 kg/h, air fuel ratio 22, room temperature 20°C and pressure at 1 bar. The fuel has a calorific value of 42 MJ/kg and contains of 15% by weight of hydrogen. Calculate: i. indicated thermal efficiency ii. volumetric efficiencyarrow_forwardTwo moles of an ideal diatomic gas is taken through the cycle shown. At the state 1, the pressure is P1 and the volume is V. If p =300 kPa and V, = 040 m', find p2, P3 and T3- Also compute W, Q. AE per cycle, and the thermal efficiency n. int isothermal Adiabatic Vị 3V1 Volume Pressurearrow_forward3. A water-tube boiler having a heating surface of 325.23 m² evaporates 6349.21 kg of water in an hour from a feed temperature of 66 °C. Boiler pressure is at 1.04 MPaa and the steam quality at the boiler outlet is 99 %. What percent of its rated Bo. Hp was the boiler developing?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY