
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
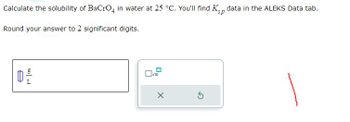
Transcribed Image Text:Calculate the solubility of BaCrO4 in water at 25 °C. You'll find Kp data in the ALEKS Data tab.
sp
Round your answer to 2 significant digits.
60)
x10
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Sodium chloride is added to water (at 25C) until it is saturated. Calculate the Cl concentration in such a solution. Species G(kJ/mol) NaCl(s) 384 Na+(aq) 262 Cl(aq) 131arrow_forwardAccording to the Resource Conservation and Recovery Act (RCRA), waste material is classified as toxic and must be handled as hazardous if the lead concentration exceeds 5 mg/L. By adding chloride ion, the lead ion will precipitate as PbCl2, which can be separated from the liquid portion. Once the lead has been removed, the rest of the waste can be sent to a conventional waste treatment facility. How many grams of sodium chloride must be added to 500 L of a waste solution to reduce the concentration of the Pb2+ ion from 10 to 5 mg/L?arrow_forwardThe hydrocarbon naphthalene was frequently used in mothballs until recently, when it was discovered that human inhalation of naphthalene vapors can lead to hemolytic anemia. Naphthalene is 93.71% carbon by mass, and a 0.256-mole sample of naphthalene has a mass of 32.8 g. What is the molecular formula of naphthalene? This compound works as a pesticide in mothballs by. sublimation of the .solid so that it fumigates enclosed spaces with its vapors according to the equation Naphthalene(s)naphthalene(g)K=4.29106(at298K) If 3.00 g solid naphthalene is placed into an enclosed space with a volume of 5.00 L at 25C, what percentage of the naphthalene will have sublimed once equilibrium bas been established?arrow_forward
- Calculate the solubility of PbCrO4 in water at 25 °C. You'll find K data in the ALEKS Data tab. Round your answer to 2 significant digits. 3012 x10 X Śarrow_forwardCalculate the solubility of ZnCO, in water at 25 °C. You'll find K data in the ALEKS Data tab. Round your answer to 2 significant digits. g_L x10 X 6arrow_forwardCalculate the solubility of PbCrO4 in water at 25 °C. You'll find K data in the ALEKS Data tab. Round your answer to 2 significant digits. 20 0x10 Xarrow_forward
- 1 4 Calculate the solubility of Fe (OH), in water at 25 °C. You'll find K data in the ALEKS Data tab. ds. Round your answer to 2 significant digits.arrow_forwardCalculate the solubility of Mg (OH), in water at 25 °C. You'll find K.n data in the ALEKS Data tab. sp Round your answer to 2 significant digits. x10arrow_forwardNonearrow_forward
- Calculate the solubility of Co(OH) 2 in water at 25 °C. You'll find Ksp data in the ALEKS Data tab. Round your answer to 2 significant digits. g ☐ x10arrow_forwardg The solubility of Mg(OH)2 in water at 25 °C is measured to be 0.0096 Use this information to calculate K for Mg(OH)2. Round your answer to 2 significant digits. 00 L 3arrow_forwardThe solubility of AgBrO, in water at 25 °C is measured to be 1.7 Use this information to calculate K for AgBrO3. sp Round your answer to 2 significant digits. x10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning