
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:1000
-40
900
32
-100
800
700
600
500
400
300
200
100
-150
10
20
30
40
50 60 70 80
90
100
Composition, Mole % Methane
Appendix 2
Pressure (psia)

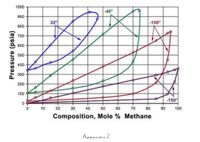
Transcribed Image Text:Calculate the quantities of gas and liquid formed when 7.5 lb moles of mixture
of 60% methane and 40% ethane are brought to equilibrium at -40 °F and 600
psia based on Appendix 1. The equilibrium tie-line should be clearly indicated
in the diagram.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Similar questions
- Please help me answer at least all of the given questions. Thank you.arrow_forward1) "Boiling Points" - Txy and xy diagrams. The vapor-pressure for the system n-octane & n-decane is available (spreadsheet): a) Using Raoult's Law and if needed Antoine's Equation calculate and plot the Txy diagram for a total pressure of 101.325kPa. b) Plot the xy data for this system at a total pressure of 101.325kPa.arrow_forward1) Ethanol-Water Separations. We wish to separate ethanol from water in a sieve-plate distillation column with a total condenser and a partial reboiler. There are two feed streams: Thermal State subcooled liquid* saturated vapor *Feed 2 condenses 0.25 moles of vapor for every mole of feed. The bottoms product should be 2% (mol) ethanol and the distillate should be 72% (mol) ethanol. Feed Flowrate (mol/hr) ZF 1 0.4 2 0.3 200 300 Notes: • The reflux ratio is equal to (1.0) and the feeds are to be input at their optimum location(s). • Both feeds are being input into the column, e.g. this is not intended to be solving for two unique columns but just one that has two input feed streams. • Equilibrium data for Ethanol-Water at 1 bar is shown in the table. You may also identify /use other experimental data (web sources, library) for this system. a) What are the flowrates of the distillate and bottoms products? b) What are the flowrates of liquid and vapor on stages between the two feeds…arrow_forward
- 1. Use the Chapman-Enskog theory and Fuller correlation to estimate the diffusion of hydrogen in nitrogen at 21°C and 2 atmospheres.arrow_forwardEthylene gas is converted ethanol compound by vapor phase hydration reaction. is being transformed. Maximum conversion amount at 523 K and 35 bar conditions, Calculate based on the amount of steam being 5 times the amount of ethylene. gas in equilibrium Assume that the mixture exhibits ideal solution behavior.arrow_forwardDraw a Pxy diagram for an aqueous solution of NH3. The temperature is such that the vapor pressure of the pure water is 5 kPa and the vapor pressure of pure NH3 is 600 Pa. You need to include the bubble line and the dew 7. line. (a) What is the vapor pressure of the solution when rNH, = 0.6? (b) In which phase the solution will be at P = 4.8 kPa? (c) In which phase the solution will be at P =0.2 kPa? (d) Draw the path for a process that takes a liquid with INH, = 0.6 %3D and P= 4.8 kPa to P= 0.2 kPa.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The