
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
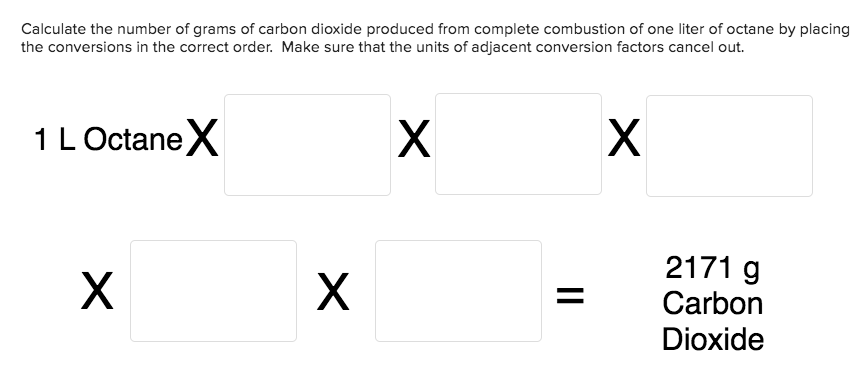
Transcribed Image Text:Calculate the number of grams of carbon dioxide produced from complete combustion of one liter of octane by placing
the conversions in the correct order. Make sure that the units of adjacent conversion factors cancel out.
X
X
1 LOctaneX
2171 g
X
Carbon
Dioxide

Transcribed Image Text:8 mol CO2
114 g Octane
1000 mL Octane
1 mol Octane
1 mol Octane
8 mol CO2
1 mol Octane
1 L Octane
44 g CO2
1 mol Octane
1 mL Octane
0.703 g Octane
114 g Octane
1 mol CO2
0.703 g Octane
1 mL Octane
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Can someone help me out with the conversion aspect of this question? Im getting the answer right but the conversion wrongarrow_forwardConsider the following balanced equation: 2 H2 + O2 → 2 H2OWhat mass of water (H2O) will be collected if 60.0 grams of oxygen gas (O2) are consumed? (Mass of H2O = 18.02 g/mol and Mass of O2 = 32.00 g/mol)arrow_forwardCH4(g)+2 O2(g) → CO2(g)+2 H2O(g)+energy What type of reaction is this? Single displacement Combustion Combination Double displacement Decompositionarrow_forward
- Use 1 decimal point for all atomic masses. 12.3 g of NCl3(g) are reacted with 0.605 g of H2(g) by the following reaction NCl3(g) + 3H2(g) --> NH3(g) + 3HCl(g) What is the limiting reagent? NCl3(g) H2(g) Based on the limiting reagent, what should the yield of NH3(g) be? garrow_forwardConsider the following balanced equation. 3 Ag(s) + 4 HNO3(aq) → 3 AgNO3(aq) + NO(g) + 2 H2O(l) A student used 117.24 g of Ag and obtained 135.49 g of AgNO3, calculate the percent yield of AgNO3. Give your answer to the correct number of significant figures without unit. Molar mass of Ag: 107.87 g/mol Molar mass of HNO3: 63.01 g/mol Molar mass of AgNO3: 169.87 g/mol Molar mass of NO: 30.01 g/mol Molar mass of H2O: 18.02 g/molarrow_forwardQ-garrow_forward
- Assume that your empty crucible weighs 15.98 g, and the crucible plus the sodium bicarbonate sample weighs 18.56 g. After the first heating, your crucible and contents weighs 17.51 g. After the second heating, your crucible and contents weighs 17.50 g. Answer the following questions based on this information.arrow_forwardClassify each chemical reaction.arrow_forwardIdentify/label each chemical reaction according to the types listed in the table below by dragging the correct responses into the blanks. Chemical Reactions: 12FeCl2(s) + 3O2(g) → 8FeCl3(s) + 2Fe2O3(s) Br2(l) + C2H4(g) → BrCH2CH2Br(l) HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l) CaCl2(aq) + K2SO4(aq) → CaSO4(s) + 2KCl(aq) Types of chemical reactions: redox reaction exchange acid-base condensation. Please give Typed answer not hand written .arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY