
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Consider the following diagram of reactant particles:
If these reactants undergo the reaction described by the equation
A2 (g) + 4 B (g) → 2 AB2 (g),
How many AB2 molecules will be made?
Answer as an integer. Do not include a unit or label.
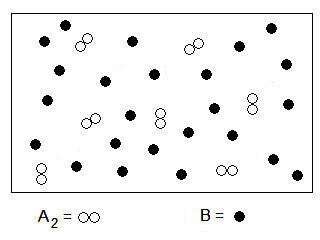
Transcribed Image Text:8
8
8
B =
A2 00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The theoretical yield of CCl4 is 50 g. If the actual yield from the reaction is 38 g of CCl4, what is the percent yield of CCl4? PY= (Actual/Theoretical)x 100arrow_forwardConsider the following unbalanced chemical equation: Fe3O4 (aq) + CO (g) → CO2 (g) + Fe (s) What will be the coefficient in front of CO in the balanced chemical equation? If there is no coefficient in front of CO in the balanced equation, enter 1 as the answer.arrow_forward[Tutorial: Limiting reactant stoichiometry] This question will walk you through the steps of calculating the mass of products produced based on your determination of the limiting reactant. b) Step 2a: Use dimensional analysis to determine the theoretical yield of the product. Calculate the theoretical yield in grams Al₂O₃ from the complete reaction of 64.7 grams Al according to the following balanced chemical equation: 2 Al(s) + Fe₂O₃(s) → Al₂O₃(s) + 2 Fe(s) c) Calculate the theoretical yield in grams Al₂O₃ from the complete reaction of 201 grams Fe₂O₃ according to the following balanced chemical equation: 2 Al(s) + Fe₂O₃(s) → Al₂O₃(s) + 2 Fe(s) d) Which of the following substances is the limiting reactant? e) What is the mass in grams of the excess Fe₂O₃ remaining after the partial reaction of 201 g Fe₂O₃ with 64.7 g Al? Give your answer to three significant figures.arrow_forward
- Cobalt reacts with water according the the equation below. When 15.66 grams of cobalt is added to an excess amount water, how many moles of cobalt(III) oxide will be produced by the time the reaction is 51% complete? Report your answer with three significant figures. 2 Co + 3 H2O → Co2O3 + 3 H2arrow_forward[References] Use the References to access important values if needed for this question. For the following reaction, 27.8 grams of sodium chloride are allowed to react with 66.9 grams of silver nitrate. sodium chloride ( aq ) + silver nitrate ( aq) → silver chloride ( s) + sodium nitrate ( aq ) What is the maximum amount of silver chloride that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? grams Visited Submit Answer Try Another Version 1 item attempt remaining Show Hintarrow_forwardA student knows the mass of one of the reactants of a chemical reaction and wants to calculate the mass of one of the products of the reaction. Which process should the student follow? Multiply the mass of the reactant by its molar mass to find the number of moles of the reactant. Use the chemical equation to find the number of moles of the product. Multiply the number of moles of the product by its molar mass to find the mass of the product. Multiply the mass of the reactant by its molar mass to find the number of moles of the reactant. Use the chemical equation to find the number of moles of the product. Divide the number of moles of the product by its molar mass to find the mass of the product. Divide the mass of the reactant by its molar mass to find the number of moles of the reactant. Use the chemical equation to find the number of moles of the product. Multiply the number of moles of the product by its molar mass to find the mass of the product. Divide the mass of the reactant…arrow_forward
- Consider the general chemical equation 3A+B→2C. Part A. If 1.45 g of A reacts with 1.75 g of B, what is the mass of C? Express your answer with the appropriate units. Part B. If 4.70 g of A reacts to produce 8.95 g of C, what is the mass of B? Express your answer with the appropriate units.arrow_forwardDo not give handwriting solution.arrow_forwardDetermine the theoretical yield of water, in grams, when 19.66 grams of O2 reacts according to the reaction below. Use two decimal places for your molar masses and use the abbreviation for the unit. C3H8 (g) + 5 O2 (g) → 3 CO2 (g) + 4 H2O (g)arrow_forward
- From the list of reactions below, pick out the combustion reactions? 1) 2C2H6(g) + 7O2 (g) → 4CO2(g) + 6H2Ol) 2) MgO(s) + CO2 (g) → MgCO3(s) 3) ZnCO3(s) → ZnO (s) + CO2 (g) 4) 2CH3OH(l) + 3O2 (g) → 2CO2(g) + 4H2O(l) a 1, 2, 3, and 4 b 3 and 4 c 2, 3, and 4 d 1, 3, and 4 e 1 and 4arrow_forwardSuppose the following chemical reaction can take place in this mixturearrow_forwardCalculate the theoretical yield of the product in moles for each of the initial quantities of reactants. Ti(s) + 2Cl2(g) -> TiCl4(s)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY