
World of Chemistry, 3rd edition
3rd Edition
ISBN: 9781133109655
Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher: Brooks / Cole / Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
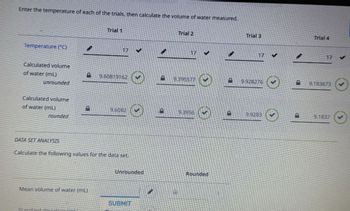
Transcribed Image Text:Enter the temperature of each of the trials, then calculate the volume of water measured.
Temperature (°C)
Trial 1
17
Calculated volume
of water (mL)
B
9.60819162
unrounded
Calculated volume
of water (mL)
Trial 2
17 ✔
9.395577
Trial 3
17 V
9.928276
Trial 4
17 ✓
9.183673
9.6082
D
9.3956
9.9283
rounded
☺
9.1837
DATA SET ANALYSIS
Calculate the following values for the data set
Mean volume of water (mL)
Standard deviation
Unrounded
Rounded
SUBMIT
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Convert 45 mi/h to m/s, showing how the units cancel appropriately.arrow_forward351145 TECHNO Define fundamental unit with an example. Why is the unit of force called a derived unit? ic a derived unit? Give its two examples. measurement Dats : D Graphite and iodine a y shine. Explain it with suitable yeasons. Graphite and are non-metals but the iodine are non- metals but REDMI NOTE 10 PRO KBYLWHARNA THAPA A chemical propertie39/99/2021 17:36arrow_forwardAbsolute zero is the temperature at which all particles motion____arrow_forward
- Temp (°C) 5.9 17.4 57.8 79.5 Data Table K 224.2 190.3 137.4 115.2arrow_forwardnHMAC%3Def41415d9158412623399163083b2ede#10001 Spr. 2021 8 Gu HI, Leira v Sign Out Item 4 I Review | Constants | Periodic Table Alexandra decides to climb Mt. Krumpett, which is 5000 m high. She determines that this will require a total of 2250 kcal of energy for the trip. For her food supply, she decides to take nutrition bars. The label states that each bar contains 50 g of carbohydrates, 10 g of fat, and 40 g of protein. Part A How many nutrition bars should Alexandra pack? Express the number of bars numerically. > View Available Hint(s) ν ΑΣφ bars Submit Previous Answers 0V 12:33arrow_forwardGrad x I Cour X G 2.02: X G 2.0 m x G A hy X G oxide x G Exerc X E 4 -M X O Bana X G 1.84 x + college.com/course.html?courseld=16985674&OpenVellumHMAC=a0b841caaee7feddab1623a6c83a92c0#10001 I Review | Constants | Periodic MISSED THIS? Watch IWE 4.13; Read Section 4.10. You can click on the Review link to access the section in your e Text. 2.0 mol CH12 Express your answer in moles to two significant figures. Determine the number of moles of hydrogen atoms in each of the following samples. .? mol Submit Request Answer Part D 2.02 mol Cg H18 Express your answer in moles to three significant figures. mol Submit Request Answer P Pearson Copyright © 2021 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy I Permissions | Contact Us I arch 3:46 88°F Mostly cloudy a a 4x 10/15/ 10 inort sc delete home end %24 4 & num 6 8 backspace lock Y U { [C home H J enter pause V B /| N M ↑ shiftarrow_forward
- A cylinder measuring 8.4 cm wide and 10. cm high is filled with gas and the piston pushed down with a steady force. - piston cylinder gas Calculate the force on the piston if the pressure in the gas is measured to be 719. kPa. Write your answer in units of kilonewtons. Round your answer to 2 significant digits. Ox10 ?arrow_forwardMeasurements of a patient's temperature are routinely done several times a day in hospitalsDigital thermometers are used, and it is important to evaluate thermometers and select the best one. The accuracy of these thermometers is checked by immersing them in liquids of known temperature. Such liquids include an ice-water mixture at 0.0and boiling water at 100.0C at exactly 1 atmosphere pressure (boiling point with atmospheric pressureSuppose the data shown in the following table were obtained on three available thermometers and you were asked to choose the "best one of the three Which would you choose? Explain your choicearrow_forwardA cylinder measuring 2.1 cm wide and 2.5 cm high is filled with gas and the piston pushed down with a steady force. • piston cylinder gas Calculate the force on the piston if the pressure in the gas is measured to be 231. kPa. Write your answer in units of kilonewtons. Round your answer to 2 significant digits. O KNarrow_forward
- C. The Thermometer and Its Calibration 2. 98 762 Observed temperature of water-and-ice mixture °C °C -Temperature of boiling water Observed atmospheric pressure mm Hg °C True (corrected) temperature of boiling water °C Thermometer correctionarrow_forwardTemp. (°C) B. 1501 135 120 D. E, 06 75 C, 09 30 A 15 Heat (kJ) Created by E. Lee for Virtuol Virginia (2021) Using this heating curve, complete the following statements: substance's melting freezing or point be uan punoj between points | SelectJ 1. The pue | Select] (Select the points alphabetically; for example, "X and Y" not "Y and X") V and Select the substance is in the liquid phase. 2. Between points Select| (Select the points alphabetically: for example, "X and Y" not "Y and X") 3. At 117°C, the substance is a | Select J &arrow_forwardcntical point 218 water ice P. atm briple point 0.006 water Phe arge dreng vapor 100 374 0. 001 T C At a constant 220 atm, water can be depending on the temperature O solid, liquid, and gas solid, liquid, and supercritical fluid solid and liquid solid and gasarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning