
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
- C9H10O2: IR absorption at 1718 cm–1. Propose a structure with data given.
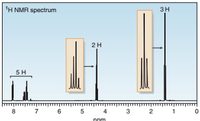
Transcribed Image Text:'H NMR spectrum
зн
2H
5 H
8
7
5
3
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Suggest structures given the 1H NMR spectra and formulas for each of the compounds below. C4H10Oarrow_forwardAn organic compound B with formula C6H14O has the following: IR Spectroscopy 2974 cm-1, 1080 cm-1 Mass Spectrometry 102 (M+), 87, 73 1H NMR Spectroscopy Eight signals at δ 1.10 (d, 3H), 1.13 (dd, 3H), 1.14 (dd, 3H), 1.59 (ddq, 1H), 1.60 (ddq, 1H), 3.19 (ddq, 1H), 3.51 (dq, 1H), 3.50 (dq, 1H). Compound B is obtained by the reaction of compound A with NaH followed by CH3CH2Br. The stereochemistry of A is "S" Using this information, deduce a plausible structure for Compound A with correct stereochemistry.arrow_forwardCan u pls explain ur answers in details thank uarrow_forward
- Spectra C 3000 200 180 160 140 120 100 BO 80 B-4-A ppm 4000 3000 2000 8. 10 40 20 11 10 9 7 6 5 3 ppm M 1000 1500 1000 500 Structure: IR: 13C NMR: • Number of types of C: ⚫ Chemical shift(s) of signals: 'H NMR: Number of types of H: Chemical shift(s) of signals: • Integrations: Multiplicity of peaks: Wavenumbers, cm N nonet 0arrow_forwardThe primary amine isoamylamine (3-methylbutylamine) occurs naturally in a number of fruits and is used as a food flavouring: (CH3),CHCH,CH,NH, 3-methylbutylamine Suggest two ways in which it can be synthesised from suitable organic precursors. Note that only one of the two methods you propose may involve the reduction of another nitrogen containing compound. In each case, you should indicate what starting material and other reagents are needed, though details of precise experimental conditions or reaction mechanisms are not required.arrow_forwardPropose a structure, carefully outlining how you arrived at that answerarrow_forward
- A From the given spectral data, identify the compound and provide justification for MS fragmentation, IR bands and identify the protons and provided NMR data : carbon from the Mol. formula : C5H100 Ms : m/z= 86 · 1, 71, 44 IR (liquid film) : 1728, 2822, 2960 (cm-) 'H-NMR (8) : A =9·75, B =2:32, C =2.21, D =0:98 ppm (suggest splitting pattern) 13 C-NMR (8) : (Proton decoupled) 1 =202.71, 2 = 52.66, 3 = 23-57, 4 = 22·59arrow_forwardReaction of (CH3)2CO with LiCCH followed by H2O gives compound X, which has a molecular ion in the mass spectrum at 84. It also has prominent absorptions in the IR spectrum at 3600-3200, 3303, 2938, and 2120 cm-1. The proton NMR shows a singlet at 1.53 (6H), a singlet at 2.37 (1H) and a singlet at 2.43 (1H). What is the structure for compound X?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY