
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The IR and 1H-NMR spectra of a compound with molecular formula C4H7ClO2 are shown below. Your objective as a group is to propose a structure for this compound, explaining how you reach your decision. Using all the information you have been given, in a post with others in your group share your initial ideas about the possible structure of the compound. Then use comments to interact with the other students in the group and propose a final answer to the problem. In the comment phase, you should comment on the postings of at least two other students.
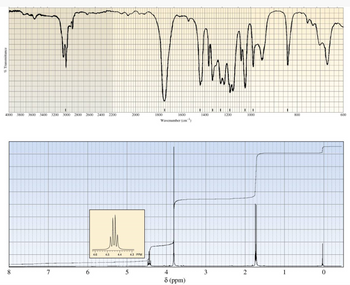
Transcribed Image Text:% Transmittance
4000
8
3800 3600 3400 3200 3000 2800 2600 2400 2200
7
16
4.6
J
4.5
4.4
5
nww
Mumm
2000
4.3 PPM
1800
1
1600
Wavenumber (cm¹)
4
8 (ppm)
1400
3
I I
I
1200
II
1000
2
1
I
800
0
600
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. Propose a structure for an organic compound with molecular formula C3H1404 given the following 'H NMR and IR spectra. Draw the structure only, no need to interpret the spectra. 1H NMR Spectrum IR Spectrum Triplet Singlet d (6H) 8 (4H) 8 (4H) 1740 cm1 Quartetarrow_forwardA compound A of mass 114 has the following elemental analysis: C = 63.14%, H=8.83%. The IR, 1H NMR and 13C NMR spectra are given below. 1) Assign and interpret all the data from the 1H NMR and 13C NMR spectra. Propose a formula for A. 7.0 175 с 3000 1,3; 13 I=1 6.0 150 I=1 с 5.0 CH₂ 125 2000 Explain why the answer is this one : CH,—CH,—0 4,2; 60 mavermy I=2 cm-1 4.0 100 1500 3.0 75 135 167 | CH₂ 6 125 CH 3 1,95 ; 18 I=3 1000 2.0 50 H 5,5 I=3 1.0 CH₂ CH₂ 25 500 Ppm ppmarrow_forward1.Predict the structure that would give the following ¹H and ¹3C-NMR spectra for C₂H5CI: 3.6 ille 9.0 40 3.5 8.0 35 3.4 3.3 CHCl3 7.0 30 6.0 سد 25 1.80 1.70 1.60 5.0 4.0 Chemical shift (8, ppm) 20 PPM 2H T 15 3.0 2.0 10 3H 1.0 5 TMS 0.0 0arrow_forward
- Shown are the H NMR spectra for 2 isomeric compounds three and four of the formula C5H10O. The IR spectrum of both have an absorption in the region of 1700 to 1730 cm-1. Provide the structure for each compound, and which hydrogen atoms give rise to the peaks in each spectrum. The peak at 7.27 ppm can be ignored, and the red numbers are integration values.arrow_forwardChapter 18 Worksheet 18-1 Properties of Light 1. The stretching frequency of a carbonyl (C=0) bond in a typical ketone is 5.15 x 1013 Hz. The vibrational energy of the molecule increases when light of this frequency is absorbed. a) What is the wavelength of this light in nanometers? b) What is the wavelength of this light in micrometers? c) Express the frequency of this light in wavenumbers. d) What is the energy (in joules) of a single photon of light at this frequency? e) What is the energy (in kilojoules) of one mole of photons at this frequency? f) Into what region of the electromagnetic spectrum does this radiation fall?arrow_forwardSuggest structures given the 1H NMR spectra and formulas for each of the compounds below. C4H10Oarrow_forward
- The IR spectrum, 1H NMR spectrum, and 13C NMR spectrum for the unknown compound with the formula C5H12O are given below. Make a structure for the unknown and assign the peaks in the spectra by the provided tables.arrow_forward12arrow_forwardIdentify the structure of the compound a having the following IR, 1H NMR spectra (integrals shown in the boxes). Label the spectra with the information (functional groups, number of protons, formula, etc.) you obtain Compound a. Formula: C7H14O Questions that may help you to decide: What is the element of unsaturation of the molecular formula? Show the math you use: What are the functional groups present in this molecule? Show all of them below. Draw at least two possible structures that have the required element of unsaturation as well as the observed functional groups. Based on the 1H NMR of compound a above, what molecular fragmentations do you see: Draw your final decision of the structure of compound a below:arrow_forward
- By examining the carbon NMR spectra of 1,1-dichlorocyclohexane and cis-1,2- dichlorocyclohexane, what TWO things would you look for to distinguish between the spectra. Use structures to explain your answer.arrow_forwardKeeping with the theme of autumn, one of Dr. Danahy’s favorite molecules is caffeic acid due to its presence in pumpkins. This structure serves as an antioxidant and is one of many found within pumpkins. Despite its name, it bears no resemblance to caffeine. Answer the following questions about caffeic acid. The carbonyl stretch for caffeic acid is unusually low for a carboxylic acid at 1646 cm-1. For reference, the carbonyl stretch for propanoic acid is 1716 cm-1. Explain why the carbonyl stretch occurs at a lower wavenumber for caffeic acid.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY