
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
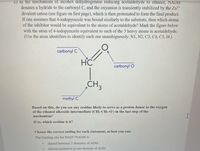
Transcribed Image Text:c) In the chanism of alcohol dehydrogenase reducing acetaldehyde to ethanol, NADH
donates a hydride to the carbonyl C, and the oxyanion is transiently stabilized by the Zn2+
divalent cation (see figure on first page), which is then protonated to form the final product.
If one assumes that 4-iodopyrazole was bound similarly to the substrate, then which atoms
of the inhibitor would be equivalent to the atoms of acetaldehyde? Mark the figure below
with the atom of 4-iodopyrazole equivalent to each of the 3 heavy atoms in acetaldehyde.
(Use the atom identifiers to identify each one unambiguously: N1, N2, C3, C4, C5, 14.)
carbonyl C
HC
carbonyl O
CH3
methyl C
Based on this, do you see any residue likely to serve as a proton donor to the oxygen
of the ethanol alkoxide intermediate (CH3-CH2-O') in the last step of the
mechanism?
If so, which residue is it?
Choose the correct ending for each statement, as best you can:
The binding site for NAD*/NADH is
shared between 2 domains of ADH.
almost exclusive in one domain of ADH.

Transcribed Image Text:shared between 2 domains of ADH.
almost exclusive in one domain of ADH.
The active site of ADH (where the chemistry takes place) is
shared between 2 domains of ADH.
almost exclusive in one domain of ADH.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 3. (а) (coenzyme A), NAD+ (oxidized nicotinamide adenine dinucleotide), FAD (oxidized flavin adenine dinu- cleotide), and a five-membered ring for oxidized lipoamide, and using structural formulas outline the reactions for the oxidative decarboxylation of a-ketoglutarate catalyzed by the aKG dehydrogenase complex. Name the 3 enzymes catalyzing the reactions underlying the conversion of a-ketoglutarate. Using the following abbreviations for cofactors: TPP (thiamine pyrophosphate), COASHarrow_forwardWhat are the amino acids of the catalytic triad of chymotrypsin? State the mode of catalysis shown by each of the amino acids that you have named. (a) (b) Predict what will happen if aspartic acid of the triad is replaced by asparagine. (c) Briefly explain the role of the oxyanion hole for the catalysis of chymotrypsin.arrow_forwardIn the pyrimidine degradative pathway, all pyrimidines undergo conversion to uracil, which undergoes an NADPH-dependent reduction. Show plausible reactions leading from cytidine, cytosine, and uridine to uracil, and write a plausible structure for the product of uracil reduction (dihydrouracil).arrow_forward
- Triosephosphate isomerase (TIM) catalyzes the conversion of dihydroxyacetone phosphate to glyceraldehyde-3-phosphate. The enzyme’s catalytic groups are Glu 165 and His 95. In the first step of the reaction, these catalytic groups function as a general-base and a general-acid catalyst, respectively. Propose a mechanism for the reaction.arrow_forwardIdentify the substrate, enzyme, and products in the following chemical reaction: HO но. HO но, HO HO OH HO Lactase NO2 он NO2 OH ONPG p-D-Galactose O-Nitrophenol o-Nitrophenol (ONP) 1. Products B-D-Galactose 2. Substrate Lactase 3. Enzyme ONPGarrow_forwardmake a schematic diagram of BChE/AChE inhibitory determination Butyrylthiocholine iodide (BChI), as well as acetylthiocholine iodide(AChI) molecules were utilized as substrate compounds of the reaction. Inthis regard, 5,5′‐dithio‐bis(2‐nitro‐benzoic acid) compound (DTNB) wasused to estimate BChE/AChE activity. In summary, 100 μL Tris buffersolution (HCl, 1.0M, pH 8.0, Tris) and variety of concentrations of thesample solution (50‐200 mL) were added in deionized water and also20 μL of BChE/AChE enzymes solutions added. The mixture was thenincubated for 10 minutes at 20°C. Finally, 50 μL of DTNB (0.5 mM and25 mL) BChI/AChI were added to the incubator mixture. The reactionwas also started by adding 50 μL BChI/AChI. The activity of theseenzymes was evaluated using spectrophotometric spectra with awavelength of 412 nm.arrow_forward
- Draw a stepwise mechanism for conversion of radical intermediate A to prostaglandin PGG2.arrow_forwardWrite balanced equations for the three known reactions that transfer an amino group to a substrate by condensation with aspartate to give an intermediate that then undergoes a,B-elimination to give the product plus fumarate.arrow_forwardWrite a balanced equation for cach of the following reactions or reaction sequences. (a) The reaction catalyzed by PFK-2 (b) The conversion of 2 moles of oxaloacetate to glucose (c) The conversion of glucose to UDP-Gle (d) The conversion of 2 moles of glycerol to glucose (e) The conversion of 2 moles of malate to glucose-6-phosphatearrow_forward
- The purinosome contains enzymes that convert the serine hydroxymethyl group to the formyl group of 10-formyltetrahydrofolate. Write a balanced equation for each reaction in this conversion.arrow_forwardCarboxylic acids, such as the one shown below, can be difficult to reduce Provide the name of a reductant A. Explain why this reductant is able to react with the carboxylic acid whereas others cannot (Hint: Draw and discuss the potential reactive intermediates/transition states).arrow_forward5) A certain aerobic organism is able to metabolize the following glycolipid: "CH,OH OH HO OH A. Draw the 2 resulting structures that would occur upon initial hydrolysis of the O-glycosidic bond. B. Calculate how much ATP is formed upon complete aerobic oxidation of one mole of the compound. Assume that no ATP is produced when one mole of the glycosidic bond in the above compound is hydrolyzed. Show calculation below.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON