
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
I would like to see the steps (arrows) that was taking to get all five isomers that is currently displayed separated with their own mechanism and reaction.
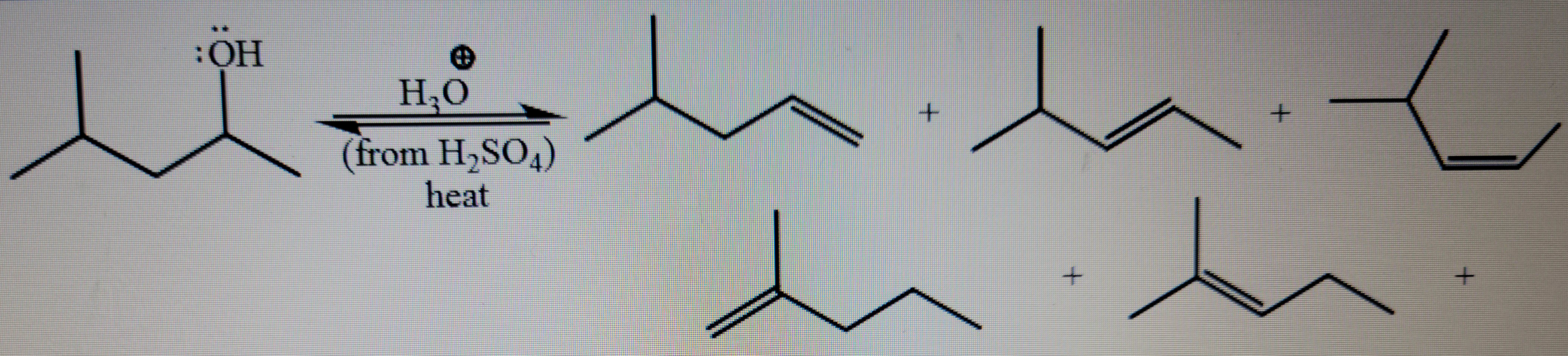
Transcribed Image Text::OH
H,O
(from H,SO4)
2
heat
+.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 11arrow_forward3) Starting from any alkene, show how you could arrive at molecular entities containing the corresponding functional groups aldehyde, alkyne or ester.arrow_forwardprovide the synthesis of Oleic acid from any straight chain alkanes containing 5 carbons or less. must make all reactants containing carbon from a straight chain alkane containing 5 carbons or less, but may utilize any reagents or solvents needed without making them.arrow_forward
- Draw a structural formula of an alkene or alkenes (if more than one) that undergo acid-catalyzed hydration and without rearrangement give 1-methylcyclohexanol as the major product. You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. ● If more than one structure fits the description, draw them all. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu. ● ● ✓ ? ChemDoodleⓇ n [ ]#arrow_forwardI am slightly confused on the naming of ethers. How come for chloromethoxymethane, it is not methoxychloride? Isn't chloride more complex than the alkyl group, so it should be placed at the root? Or is it not placed at the root because chloride is not an alkyl group at all?arrow_forwardK Draw the major product of this reaction. Ignore inorganic byproducts. x H₂O* Drawing Problem 310 of 20 Drawing Atoms, Bonds and Rings Draw or tap a new bond to see s suggestions Charges Draw ethylcycloheptane in a structural condensed format. < Draw the structural condensed formula of 1-heptyne. Drawing Problem 365 of 20 Draw a terminal alkene that would lead to this product under these conditions. Question 15 of 20 Drawing H₂ Pd/C Problem 297 of 20 Atoms Bonds and Rings Provide the correct common name for the skeletal (line- bond) structure shown here. Suarrow_forward
- producto principal HO CH3 HNO3 H2504 NO₂arrow_forwardThe careful choice of an appropriate solvent will play a major role in whether organic reactions will be successful. For example, reagents such as CH3CH2Li and NaNH2 will be incompatible with solvents such as water and ethanol. Why is this?arrow_forwardDraw a structural formula for 2-methylbutanoic acid. O • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. 8 CH4 #[ ] در ? ChemDoodlearrow_forward
- dont provide handwritinng solution...arrow_forwardDraw the major organic product(s) for the following reactionarrow_forwardplease help with the following OChem question 1. Provide the bond line structures and the IUPAC names for the major organic products formed in each step of the following reaction scheme (see attached image)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY