
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
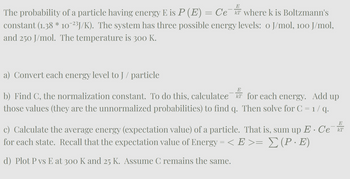
Transcribed Image Text:E
T
The probability of a particle having energy E is P (E) = Ce¯ where k is Boltzmann's
constant (1.38 * 10¯²³J/K). The system has three possible energy levels: o J/mol, 100 J/mol,
and 250 J/mol. The temperature is 300 K.
a) Convert each energy level to J / particle
E
b) Find C, the normalization constant. To do this, calculatee T
those values (they are the unnormalized probabilities) to find q.
for each energy. Add up
Then solve for C = 1 / q.
E. Ce
E
c) Calculate the average energy (expectation value) of a particle. That is, sum up E · Cе¯kT
for each state. Recall that the expectation value of Energy = < E >= Σ (P · E)
d) Plot P vs E at 300 K and 25 K. Assume C remains the same.
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- can you please help with part darrow_forwardCalculate the momentum of an X-ray photon with a wavelength of 0.17nm. How does this value compare with the momentum of a free electron that has been accelerated through a potential difference of 5000 volts? (Hint: electron mass, m, = 9.10938 x 10" kg; electron charge e = 1.602 x 10"C; speed of light e = 3.0 x 10* m.s'; 1.00 J= 1.00 VC; h = 6.626 x 10"J.s. The various energy units are: 1 J= 1 kg.m°s³, 1.00 eV =1VC, leV= 1.602 x 10"J, 1J= 6.242 x 10" eV, etc.). %3Darrow_forwardGiven the following probability distribution for a discrete random variable X; X = x 1 4 P(X = x) 2k 3k 6k 9k a) Calculate the value of k. b) Find P(X 2 2.5) CSarrow_forward
- 4arrow_forwardNote:- • Do not provide handwritten solution. Maintain accuracy and quality in your answer. Take care of plagiarism. • Answer completely. • You will get up vote for sure.arrow_forward5) Richard Feynman called the Euler relation the most remarkable formula in mathematics. Use the Euler relation to give the value of the following quantities. eio=1 ein/2_ ein = ei2π = (Hix = (rcosx = i sin x)arrow_forward
- Hand written explanationarrow_forwardCalculate the energy of a photon of light (in Joules) with a wavelength of (4.5000x10^2) nm. Use a value of (3.0000x10^8) for the speed of light. Remember 1 m (1.000x10^9) nm h = (6.63x10^-34) Js %3D Do not include units with your answer. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: х10 Answerarrow_forwardFind the uncertainty ox in x as a function of the uncertainties ou and oy in u and v for the following functions: (u+v) (а) х %3 2 (b) x =uv? (c) x= u? +v?arrow_forward
- Show your all calculationarrow_forward1:51 PM Sun Oct 9 -1/2 5) For a particle on a ring with the wavefunction: () = π kinetic energy of the particle using the operator K = -1/2 Y(0) = IT 21T K=-ħ²d² 21d² 2 cos ($) = kdo T (17¹/2)² 211 - f = "Cos (0) . # de TỈ Cos (O). t d. TỈ Cosco) do = 21 0 21 cos() calculate the average cos(0). -ħ² d² do 21 do² 10:52 @ 79% + : 5 9 +arrow_forwardThe energy equation for a particle in a cubic box of dimensions Lx = L, = Lz is Eux, w. = 8 mL? For a particle in a cubic box, how many degenerate energy levels have energy equal to 14 h/8 mL? 1 12 8. 3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning