
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
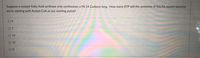
Transcribed Image Text:Suppose a mutant Fatty Acid synthase only synthesizes a FA 14 Carbons long. How many ATP will the synthesis of this FA require (assume
we're starting with Acetyl-CoA as our starting point)?
0 6
O 14
O 12
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- How many NADH, FADH2, NADPH and ATP will be generated when breaking this fatty acid down into molecules of Acetyl-CoA (16:1Δ9)?arrow_forwardThe primary purpose of the aconitase step in Citric Acid Cycle is to: form the intermediate aconitate prepare the citrate molecule for oxidative decarboxylation act as a commitment and regulatory step produce isocitratearrow_forwardin the synthesis of glycogen, the glycogen synthase uses UDP-glucose rather than glucose. Explain in detail way.arrow_forward
- The Krebs cycle reaction shown below is catalyzed by __ enzyme and ___ pays for this reaction note only major metabolites are shown a. Synthetase, hydrolysis of acetyl CoA b. Synthase, hydrolysis of acetyl CoA c. Synthase, hydrolysis of NADH d. Synthetase, hydrolysis of ATParrow_forwardIf cells synthesizing glucose from pyruvate are exposed to co2 labbeled 14C use the structure of gluconeogenesis to write where the carbon ultimately ends up.arrow_forwardFatty acid oxidation for energy yield occurs in the mitochondrial matrix, and for palmitate as one example, generates [FADN2, NADH, and Acetyle-CoA or FADH, GTP, and NADPH] , all of which can be converted to ATP (108 of them to be exact) via oxidative phosphorylation. This is a [greater or smaller] yield of ATP per carbon atom compared to glucose. Ketogenesis is a process where acetyl-CoA (including that from breakdown of fatty acids) is converted to ketone bodies under conditions where carbohydrates are [in excess or liminting] . Acetoacetate and D-beta-hydroxybutyrate are delivered from liver to the blood stream where they provide energy for [cardiac and skeletal muscle as well as brain or synthesis of glycogen] . [Insulin or glucagon] promotes ketogenesis by stimulating fatty acid export from adipose tissue.arrow_forward
- trace the position of the elctron as this glucose molecule proceeds through glycolsis and two turns of the citric acid cyclearrow_forwardPart Barrow_forwardConsidering the complete oxidation of an 18-C fatty acid. Give the answer to the following question.a. What is the total number of NADH produced in TCA if all of the acetyl CoA enters the cycle? b. What is the total number of FADH2 produced in TCA if all the acetyl CoA enters the cycle?c. How much ATP is produced in the overall oxidation?arrow_forward
- Assume that the following fatty acid is synthesized from acetyl-CoA in the cytosol by fatty acidsynthase. Which of the following statements is true? The last two carbon atoms that are added to the fatty acid are carbon atoms #15 and #16 Synthesis of the fatty acid needs 8 molecules of NADPH + H+ Both A and B Neither A nor Barrow_forwardFatty acid degradation stimulates the citric acid cyclethrough the activation of pyruvate carboxylase by acetylCoA. Why would the activation of pyruvate carboxylaseincrease energy generation from fatty acids?arrow_forwardAcetyi CoA Oxaloncetate CoA NADH NAD: Citrate Isccitrate Malate NAD co NADH Funaate »FADH; FAD a- Ketoglutarate Succinate NAD ATP Succinyt CuA NADH ADP - P, If you were told to add one of the eight citric acid cycle intermediates to the culture medium fo yeast growing in the laboratory, what do you think would happen to the rates of ATP and carbon dioxide production? (see the above figure) a. There would be no change in ATP production, but the rate of CO2 production would increase. b. The rates of ATP production and CO2 production would both increase, c. The rate of ATP production would increase, but the rate of CO2 production would decrease. d. The rates fo ATP and CO2 production would both decrease.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON