
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
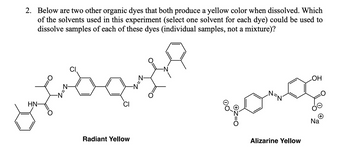
Transcribed Image Text:2. Below are two other organic dyes that both produce a yellow color when dissolved. Which
of the solvents used in this experiment (select one solvent for each dye) could be used to
dissolve samples of each of these dyes (individual samples, not a mixture)?
HN-
Radiant Yellow
Alizarine Yellow
OH
Na
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- If you wanted to separate the two dyes from each other, how would you accomplish this? Suggest a procedurearrow_forward6. (Chapter 15 - 47b) An aromatic compound, C9H12 has the following H NMR spectrum and a peak at 750 cmt in its IR spectrum. Answer the following questions. Chem. shift Rel. area 1.19 2.31 2.64 7.13 1.50 1.50 1.00 2.00 TMS 10 3 O ppm Chemical shift (6) 4(a). The IR peak at 750 cm in its IR spectrum indicate that the compound is. disubstituted = 4(b) The name of the compound is = Intensity-arrow_forwardPlease answer ALL parts of the following related to organic chemistry.arrow_forward
- Solvent #1 is a 50:50 mixture of hexane and ethyl acetate and solvent #2 is 25% hexane. Which solvent mixture is more polar: the 50:50 mixture or the 25:75 mixture? Explain briefly What would happen to the Rf of the spots in solvent #2 compared to solvent #1? Why?arrow_forwardDispersion Dye, Acid Dye, and Direct Dye Which dye will be the most polar? The least polar? Explain.arrow_forwardWhich tracking dye is used in SDS-PAGE? O bromothymol blue O bromophenol blue O coomassie blue O acridine dyearrow_forward
- 1015MSC Chemistry of Biological Systems II 2021 2. What kind of results do you expect to see when the following compounds are mixed together with the given test solution? Indicate positive or negative in the table below. (a) with 2,4-dinitrophenylhydrazine (b) with Benedict's reagent (c) with lodoform reagent CH3 (d) with Tollen's reagentarrow_forwardA student was trying to determine the identity of an unknown compound. The melting point of the compound was 117-118oC. The student narrowed the possible compound down to the following: Compound Melting Point Range acetanilide 113-115oC fluorene 114-116oC mandelic acid 120-122oC A second student, doing the same experiment as above, found that their melting point was 100-108oC. What is wrong with the student’s melting point? Propose two reasons why the melting point could be incorrect. View keyboard shortcutsarrow_forward1B. Can 1H NMR spectroscopy be used to differentiate between the two compounds? Briefly explain why or why not. Predict the 1H NMR spectrum for each compound (include integration, multiplicity, and approximate chemical shift). Put it in data table format.arrow_forward
- 3. 'H-NMR interpretation When dissolved in CDC13, phenacetin shows 6 signals. All of the peaks are sharp, except for the signal f not below. Draw the chemical structure of phenacetin, labeling each hydrogen with an alphabetical letter. Make a larger version of the chart below (half or full page), and complete it with the missing information. Explanation of splitting patter 8 (ppm) Letter # H (integration Splitting pattern value) 7.94 f 1 Broad singlet 7.36 e 6.80 d 3.98 с 2.09 b 1.38 a Extra Credit Question 4. Describe/predict how the 'H-NMR spectrum differs for acetaminophen.arrow_forwardThe compounds you are separating are; Cholesterol/cholesterol acetate on a silica gel the solvent is ligroin:ether 9:1 Do not forget to find out if cholesterol and cholesterol acetate are colored compounds if not how will you visualize the separation of the products?arrow_forwardRhodamine B is useful dye prepared in a manner very similar to fluorescein. Due to the nitrogen donating groups, rhodamine B absorbs 550 nm light and fluoresces 580 nm light. a) What color would you expect for a rhodamine B solution? b) What is the color of the fluoresced (emitted light)?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning