
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
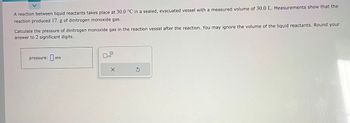
Transcribed Image Text:A reaction between liquid reactants takes place at 30.0 °C in a sealed, evacuated vessel with a measured volume of 30.0 L. Measurements show that the
reaction produced 17. g of dinitrogen monoxide gas.
Calculate the pressure of dinitrogen monoxide gas in the reaction vessel after the reaction. You may ignore the volume of the liquid reactants. Round your
answer to 2 significant digits.
atm
pressure:at
x10
X
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nitrogen monoxide gas reacts with oxygen gas to produce nitrogen dioxide gas. What volume of nitrogen dioxide is produced from the reaction of 1 L nitrogen monoxide gas with 3 L oxygen gas? What volume, if any, of the reactants will remain after the reaction ends? Assume all volumes are measured at the same pressure and temperature.arrow_forwardA 1.0-L flask contains 10.0 g each of O2 and CO2 at 25 C. (a) Which gas has the greater partial pressure, O2 or CO2, or are they the same? (b) Which molecules have the greater rms speed, or are they the same? (c) Which molecules have the greater average kinetic energy, or are they the same?arrow_forwardButane, C4H10, is an easily liquefied gaseous fuel. Calculate the density of butane gas at 0.857 atm and 22C. Give the answer in grams per literarrow_forward
- Hydrogen azide, HN3, decomposes on heating by the following unbalanced equation: HN3O(g)N2(g)+H2(g) If 3.0 atm of pure HN3(g) is decomposed initially, what is the final total pressure in the reaction container? What are the partial pressures of nitrogen and hydrogen gas? Assume the volume and temperature of the reaction container are constant.arrow_forwardA chemist weighed out 5.14 g of a mixture containing unknown amounts of BaO(s) and CaO(s) and placed the sample in a 1.50-L flask containing CO2(g) at 30.0C and 750. torr. After the reaction to form BaCO3(s) and CaCO3(s) was completed, the pressure of CO2(g) remaining was 230. torr. Calculate the mass percentages of CaO(s) and BaO(s) in the mixture.arrow_forwardA balloon filled with helium gas is found to take 6 hours to deflate to 50% of its original volume. How long will it take for an identical balloon filled with the same volume of hydrogen gas (instead of helium) to decrease its volume by 50%?arrow_forward
- A certain flexible weather balloon contains helium gas at a volume of 855 L. Initially, the balloon is at sea level where the temperature is 25C and the barometric pressure is 730 torr. The balloon then rises to an altitude of 6000 ft, where the pressure is 605 torr and the temperature is 15C. What is the change in volume of the balloon as it ascends from sea level to 6000 ft?arrow_forwardLiquid oxygen was first prepared by heating potassium chlorate, KClO3, in a closed vessel to obtain oxygen at high pressure. The oxygen was cooled until it liquefied. 2KClO3(s)2KCl(s)+3O2(g) If 171 g of potassium chlorate reacts in a 2.70-L vessel, which was initially evacuated, what pressure of oxygen will be attained when the temperature is finally cooled to 25C? Use the preceding chemical equation and ignore the volume of solid product.arrow_forwardDichlorine oxide is used as bactericide to purify water. It is produced by the chlorination of sulfur dioxide gas. SO2(g)+2Cl2(g)SOCl2(l)+Cl2O(g)How many liters of Cl2O can be produced by mixing 5.85 L of SO2 and 9.00 L of Cl2? How many liters of the reactant in excess are present after reaction is complete? Assume 100% yield and that all the gases are measured at the same temperature and pressure.arrow_forward
- Urea (H2NCONH2) is used extensively as a nitrogen source in fertilizers. It is produced commercially from the reaction of ammonia and carbon dioxide: 2NH3(g)+CO2(g)PressureHeatH2NCONH2(s)+H2O(g) Ammonia gas at 223C and 90. atm flows into a reactor at a rate of 500. L/min. Carbon dioxide at 223C and 45 atm flows into the reactor at a rate of 600. L/min. What mass of urea is produced per minute by this reaction assuming 100% yield?arrow_forwardRaoul Pictet, the Swiss physicist who first liquefied oxygen, attempted to liquefy hydrogen. He heated potassium formate, KCHO2, with KOH in a closed 2.50-Lvessel. KCHO2(s)+KOH(s)K2CO3(s)+H2(g) If 75.0 g of potassium formate reacts in a 2.50-L vessel, which was initially evacuated, what pressure of hydrogen will be attained when the temperature is finally cooled to 25C? Use the preceding chemical equation and ignore the volume of solid product.arrow_forwardYou have a gas, one of the three known phosphorus-fluorine compounds (PF3, PF3, and P2F4). To find out which, you have decided to measure its molar mass. (a) First, yon determine that the density of the gas is 5.60 g/L at a pressure of 0.971 atm and a temperature of 18.2 C. Calculate the molar mass and identify the compound. (b) To check the results from part (a), you decide to measure the molar mass based on the relative rales of effusion of the unknown gas and CO2. You find that CO2 effuses at a rate of 0.050 mol/min, whereas the unknown phosphorus fluoride effuses at a rate of 0.028 mol/min. Calculate the molar mass of the unknown gas based on these results.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER