
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
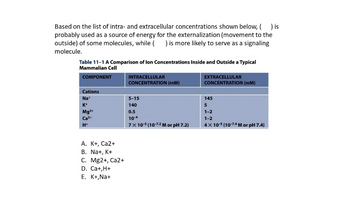
Transcribed Image Text:Based on the list of intra- and extracellular concentrations shown below, ( ) is
probably used as a source of energy for the externalization (movement to the
outside) of some molecules, while ( ) is more likely to serve as a signaling
molecule.
Table 11-1 A Comparison of lon Concentrations Inside and Outside a Typical
Mammalian Cell
COMPONENT
Cations
Na+
K+
Mg2+
Ca²+
H+
A. K+, Ca2+
B. Na+, K+
C. Mg2+, Ca2+
D. Ca+,H+
E. K+, Na+
INTRACELLULAR
CONCENTRATION (MM)
5-15
140
0.5
10-4
7 x 10-5 (10-7-2 M or pH 7.2)
EXTRACELLULAR
CONCENTRATION (mm)
145
5
1-2
1-2
4x 10-5 (10-7-4 M or pH 7.4)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- The following table shows experimental results of the glucose transport rate, mM/sec, following facilitated diffusion by glucose carrier proteins. (Recall: the starting conc. L represents glucose added to one side of the membrane; distilled water, omM of glucose was added to the other side of the membrane). The rate of glucose transport was 0.0031 mm/sec with 8mM of glucose (run number 4, highlighted); the rate decreased to 0.0017 mM/sec with 10mM of glucose (run 5, highlighted). Why was the rate of glucose transport slower when the concentration gradient was increased? Experiment Results Run Number Solute 1 1 2 2 3 33 4 4 5 6 6 Na Ch Glucose Na Ch Glucose Na Ch Glucose Nat Ch Glucose Na Ch Glucose Nat Cl Glucose Start Conc. L Start Conc. R (MM) (mM) 0.00 0.00 2.00 0.00 0.00 0.00 8.00 0.00 0.00 0.00 2.00 0.00 0.00 0.00 8.00 0.00 0.00 0.00 10.00 0.00 2.00 0.00 2.00 0.00 Carriers 500 500 500 500 700 700 700 700 100 100 700 700 Rate (mm/sec) 0.0000 0.0008 0.0000 0.0023 0.0000 0.0010…arrow_forwardGive typed full explanationarrow_forwardMatch each SERCA domain to its role in Ca2+ transport: Drag and drop options on the right-hand side and submit. For keyboard navigation... SHOW MORE ✓ N Domain P Domain A Domain ||| = = contains the Asp reside that can be phosphorylated Phosphatase activity Kinase Activity X X Xarrow_forward
- Which tonicity is optimal for animal cells and why?arrow_forward1. (a) In class thus far, we have focused our membrane transport energetics discussions on the transfer of K+ ions. Of course, in the cell there are other ions that contribute to the overall resting membrane potential (Autotal). To estimate the overall resting membrane potential for the predominant ions present in the cell, we must first calculate the individual resting membrane potential. Using the Nernst equation discussed in class, and the values provided below, calculate A for each ion. lon K+ Na+ Ca²+ CI- [ion] outside cell 6 mM 145 mM 4 mM 90 mM [ion] inside cell 145mM 8 mM 0.001 mM 6 mMarrow_forwardPls send solution fast within 10 minutes and i will give like for sure Solution must be in typed form Calcium ions bind to the SERCA Ca2+ -ATPase, which has two identical calcium sites, in two stages with apparent equilibrium constants K1=7×105M−1 and K2=2×106M−1 . a.) Calculate K. Note: K is not 0.1*1013 or 14*1011 or 1*1012. b.) Calculate τ.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON