
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Give detailed Solution with explanation needed. .don't Give handwritten answer..don't use Ai for answering this
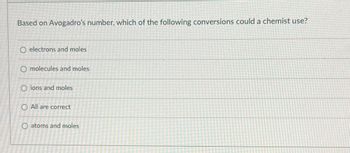
Transcribed Image Text:Based on Avogadro's number, which of the following conversions could a chemist use?
O electrons and moles
O molecules and moles
Oions and moles
O All are correct
O atoms and moles
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 1 steps

Knowledge Booster
Similar questions
- What would be the multiplicity associated with # 1, 2 & 3? Hint: Remember to think about chemically equivalent protons. a. q b. t c. d d. sarrow_forwardHow many (theoretical) peaks can you expect from the H2O in IR spectroscopy? Please shortly answer at your own words. Answer should be to the point (Specific).arrow_forward1. List the concentrations of the stock. [Acetone] 0.6M [lodine] 00025M [HC1] 2. From the computer plot of the data for each trial (absorbance vs. time in seconds), detemine the slope of the best straight line; use the following data table to summarize your results: Table 1. Trial # Acetone, mL lodine, mL HCI, mL AAbs/At ( slope of line) H20, mL A 1.0 1.0 1.0 2.0 -0.0010258xto:34978. t0.0016339x+0.44357 70,00g0217x 0.89112 0. 0013205xto,235271 2.0 1.0 1.0 1.0 1.0 2.0 1.0 1.0 D 1.0 1.0 2.0 1.0 3. Convert stock concentrations to initial concentrations and fill in Table 2: Table 2. Trial # Disappearance Rate of l2 (M/s) -A[L/At (= -slope/730) [Acetone] [lodine] [HCIJ 0.12 0.00050.12 0.24 0.12 10.12 0. coe 5 0.12 0.001 0.12 D 0.00050.24arrow_forward
- I am stuck with deriving these two equations in the far right corner in the box. i reached this far and can't seem to understand the next step moving foward. can you please derive the two equations from eqn 1,2 and 3. These are absorbance equation used in experimental determination of 2-Naphthol excited state PKa. The purpose of the formula is to find the unknown concentration of ArOH and its conjugate base in the solution given absorbance data.arrow_forwardWhat types of molecules can study in IR spectroscopy? Please shortly answer at your own words. Answer should be to the point (Specific).arrow_forwardExplain infrared spectrum in detailed analysis.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY