
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Analyze the HNMR data attached and state all take aways possible (stating all protons, chemical shift, multiplicity, integration, and coupling constants if necessary)
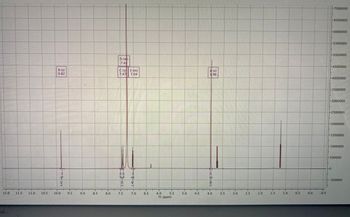
Transcribed Image Text:12.0
fol...
11.5
11.0
10.5
B (s)
9.82
10.0
EC-26'0
9.5
9.0
8.5
8.0
D (m)
C(s) E (m)
7.47
7.04
090-652
7.5
1.46 0.44
7.0
6.5
6.0
5.5
f1 (ppm)
5.0
4.5
A (S)
3.96
3.00-11.00
4.0
3.5
3.0
2.5
2.0
1.5
1.0
0.5
0.0
-0.5
-7000000
-6500000
-6000000
-5500000
-5000000
-4500000
-4000000
-3500000
-3000000
-2500000
-0
-2000000
-1500000
-1000000
-500000
--500000
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- THIS CAN NOT BE HAND - DRAWN Illustrate using a computer program the molecule from the molecular formula and interpret each of the following 1H NMR spectra. The integration for each peak is given above the peak. All spectra were taken from http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi.arrow_forwardFor the following structures: (a) find # signals, chem shift ranges, integration, splitting and (b) match the two spectrums.arrow_forwardpredict the shift for the carbon indicated by the arrows please. USE NMR SPECTRUM TABLE please.arrow_forward
- Why KBr used in IR spectroscopic sample preparation? Please shortly answer at your own words. Answer should be to the point (Specific 2-3 lines).arrow_forwardNMR data Summary NMR solvent: DMSO-d6 'H NMR: Splitting (Multiplicity) Integration Chemical Shift 9.67 singlet 1H singlet doublet 9.17 7.37 6.72 2.00 1H 2H doublet singlet 2H 3H 1"C NMR: Chemical Shift 167.8 153.3 131.1 121.1 115.1 23.8 15N NMR: Chemical Shift 132.3 Instructions: Step 0: Look at the structure of Tylenol. Determine integration, and splitting you are expecting in the 'H NMR. Step 1: Look at the integration and splitting of the signals in the H NMR spectrum - assign the chemical shift of the protons of the methyl group; make a note which signals in the 'H NMR are due aromatic protons, NH and/or OH. Step 2: Look at the 'HSN HSQC spectrum - Use this spectrum to assign the chemical shifts of the NH proton and the OH proton. Step 3: Look at the 'H1SN HMBC spectrum - Use this spectrum to assign the chemical shifts of the aromatic protons in the 'H NMR. Step 4: Look at the 13C NMR spectrum - Make a note which carbon atoms are aromatic and/or alkyl. Assign the chemical shift of…arrow_forward3 Sketch the appearance of the A2M5X2, 1H-NMR spectrum where A, M, and X represent groups of protons with distinctly different chemical shifts and JAM >JAX >JMX. NOTE: PLEASE sketch for A2M5X2 and NOT for A2M2X5arrow_forward
- ching and le X A PLQ-PHY2054L.docx - Google X engagenow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=Dassignment-take ... Translate Open Vellum Course Home [Review Topics] [References] Splitting of a signal in a proton NMR spectrum tells us the number of chemically non-equivalent hydrogens in the immediate vicinity of the hydrogen giving the signal. Predict the number of lines exhibited by hydrogens at the labeled positions in a first-order NMR spectrum. (Make the approximation that all coupling constants are equal.) 1) b a The number of lines exhibited by hydrogen(s) a is The number of lines exhibited by hydrogen(s) b is The number of lines exhibited by hydrogen(s) c is 2) b NH, The number of lines exhibited by hydrogen(s) a is The number of lines exhibited by hydrogen(s) b is The number of lines exhibited by hydrogen(s) c is Darrow_forwardMatch the following molecules with their infrared spectrum on the next two pages by placing the answer on the provided lines. There are more molecules (six) than spectra (four). No explanation is necessary. NH₂ % Transmittance % Transmittance 100 90 80 70 60 50 40 30 100 90 80 70 60 50 40 30 20 II OH 4500 4250 4000 3750 3500 3250 III IV 3000 2750 2500 2250 2000 1750 1500 1250 1000 750 500 Wavenumbers (cm-1) FTIR NEAT 250 4500 4250 4000 3750 3500 3250 3000 2750 2500 2250 2000 1750 1500 1250 1000 750 500 250 Wavenumbers (cm-1) V OH VIarrow_forwardShow how the NMR spectrum of 2-bromo-2-methylbutane would look like, include approximate chemical shifts, integration ratios, and signal multiplicity. Briefly state your reasoning so i can understand better, thanks!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY