
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Give detailed mechanism Solution with explanation needed. don't give Ai generated solution...choose the reaction conditions to complete the acid-base reaction.
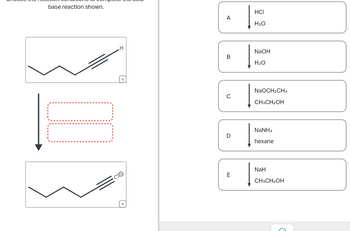
Transcribed Image Text:base reaction shown.
C:O
A
HCI
H2O
H
B
NaOH
H2O
NaOCH2CH3
с
CH3CH2OH
NaNH2
D
hexane
E
NaH
CH3CH2OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- The key step in a reported laboratory synthesis of sativene, a hydrocarbon isolated from the mold Helminthosporium sativum, involves the following base treatment of a keto tosylate. What kind of reaction is occurring? How would you complete the synthesis?arrow_forwarddraw detailed arrow pushing mechanism for these reaction.arrow_forwardSummarize the relationship between pKa and acid strength by completing the following sentences: a. The higher the pKa of an acid, the stronger or weaker the acid. b. The lower the pKa of an acid, the stronger or weaker the acid.arrow_forward
- 1. MeOH (1 eq), H* 2. nBuOH (1 eq), H*arrow_forwardCoumarin, a naturally occurring compound isolated from lavender, sweet clover, and tonka bean, is made in the laboratory from o hydroxybenzaldehyde by the reaction depicted below. Draw a stepwise mechanism for this reaction. Coumarin derivatives are useful synthetic anticoagulants.arrow_forwarddraw and complete reactionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning