
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please answer 17 and 18.
Transcribed Image Text:O B.
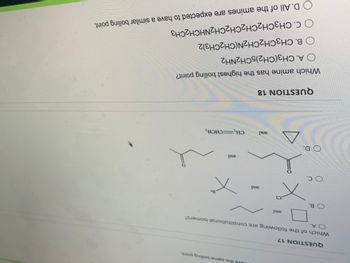
QUESTION 17
O A.
Which of the following are constitutional isomers?
O C.
and
OD.
and
and
ve the same boiling point
QUESTION 18
X
and
CH₂=CHCH₂
Which amine has the highest boiling point?
O A. CH3(CH2)5CH2NH2
B. CH3CH2CH2N(CH2CH3)2
O C. CH3CH2CH2CH2CH2NHCH2CH3
D. All of the amines are expected to have a similar boiling point.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (Pages 327 -330) this problem. Enter the formula for the conjugate acid for each of the following bases.arrow_forwardEDTA is a hexaprotic system with the pK, values: -ooc-CH2 H2C-coo pKal = 0.00, pKa2 = 1.50, pKa3 = 2.00, pK4 = 2.69, :N-CH2-CH2-N: pKas = 6.13, and pKa6 = 10.37. "ooc-CH2 H,C-coo The distribution of the various protonated forms of EDTA EDTA (or Y4-) will therefore vary with pH. For equilibrium calculations involving metal complexes with EDTA, it is convenient to calculate the fraction of EDTA that is in the completely unprotonated form, Y*-. This fraction is designated ayt-, Calculate ayt- at two pH values. pH = 3.70 ayt- = pH = 10.45 ay4- =arrow_forward4) Draw a titration curve for the tetrapeptide Trp-Asp-Lys-Gly. Label all pK,s, the pl and the net molecular charges at pH 1 and pH 14 on the graph with their values.arrow_forward
- Which type of mass analyzer is used in the MS analysis of large molecules like proteins? O Quadrupole O Magnetic Sector Time of Flight Olon Traparrow_forwardPlease answer fast i give you upvote.arrow_forwardQ: 1.5%. Sucrose by massx M what volume f soft,dmink Solin Noitolu millimeter contain' 5:29 of Suvrose bolovitine 102 102 24A) nioino) 220 ud s d ro tnaroa 220M 220m Varrow_forward
- Can you please label all function groups in this drug. Is this drug considered an isotope? what make it an isotope?arrow_forwardExplain a possible scenario at the molecular level that could explain how CML relapse could be independent of Gleevec resistance.arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY