
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
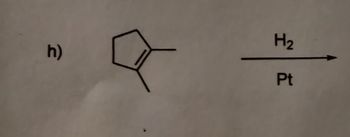
Transcribed Image Text:h)
H₂
Pt
Expert Solution
arrow_forward
Step 1: Defining addition reaction
Answer:
Addition reaction is the type of reaction in which addition of new atoms or groups takes place in the reactant molecule without the removal of any other existing atom or group.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the productarrow_forwardNaOH Ph 'Ph Δ Brarrow_forwardAspirin can be synthesized in the lab by combining salicylic acid (C,H,0,) and acetic anhydride (C,H,O,) to form aspirin ( C, H,O,) and acetic acid (C,H,O,). The balanced equation for this reaction is C,H,O, + C,H,O, → C,H¿O4 + C,H,O, A student started with 4.02 mL acetic anhydride (density of aspirin. = 1.08 g/mL) and 2.41 g salicylic acid. The student synthesized 2.41 g Select the limiting reactant. acetic anhydride (C,H,O,) salicylic acid (C,H,0,) O acetic acid (C,H,O,) aspirin (C,H¿O,) Calculate the theoretical yield of aspirin (C,H,O4). theoretical yield: g Calculate the percent yield for aspirin (C,H,O4). percent yield:arrow_forward
- Much of the sulfur used in the United States comes from the hydrogen sulfide contaminant that makes "sour" natural gas smell bad. Hydrogen sulfide is separated from the other components of natural gas mostly by taking advantage of its acid-base reaction with aqueous ethanolamine: HO(CH,),NH,(aq)+H,S(g) → HO(CH,),NH,(aq)+HS (aq) Suppose an engineer decides to study the rate of this reaction. She prepares four reaction vessels with 167.6 mL of ethanolamine solution and 20.9 g of hydrogen sulfide gas each. The volume and temperature of each vessel is shown in the table below. Arrange the reaction vessels in decreasing order of initial rate of reaction. In other words, select a "1" next to the vessel in which the engineer can reasonably expect the initial rate of reaction to be highest, a "2" next to the vessel in which the initial rate of reaction would be next highest, and so on. vessel volume temperature A B C D 5.0 L 5.0 L 5.0 L 5.0 L 49, °C 48. °C 51. °C 50. °C X initial rate of…arrow_forwardConsider the reversible reaction A + B 2 AB, shown in representation [1] at equilibrium. If representation [2] shows the system after the equilibrium has been disturbed, in which direction must the reaction be driven to achieve equilibrium again? B `A [1] [2]arrow_forwardQUESTION 10 (4b-401-1.53-1) An intern working in a research lab knocks over a beaker containing an important solution. The beaker originally contained 1.50 L of a 1.53 M solution, but after it spilled it only contained 1 L of solution. The intern decides to hide his mistake by refilling the beaker to contain 1.50 L again by adding pure water (not realizing that this will dilute the solution). What is the new concentration of the solution? Show all work and give your answer with three sig figs. Click Save and Submit to save and submit. Click Save All Answers to save all answers. Save All Answer 1600 4,104 PAGES APR O P W MacBook Air DII DD 80 000 000 F7 F8 F9 F10 F2 F3 F4 F5 F6 F1 #3 $ & 2 3 4 6 7 8. W E T Y P R ... 2.arrow_forward
- Sodium hydrogen carbonate NaHCO3, also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid HCl, which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HCl through this reaction: HCl(aq)+NaHCO3(aq)→NaCl(aq)+H2O(l)+CO2(g) The CO2 gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 200.mL of a 0.089M HCl solution. What mass of NaHCO3 would she need to ingest to neutralize this much HCl? Be sure your answer has the correct number of significant digits.arrow_forwardDd.101.arrow_forwardOne way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(II) carbonate, in concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copper(II) sulfate. Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: Fe(s)+CuSO4(aq)→Cu(s)+FeSO4(aq) Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 350.mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 142.mg. Calculate the original concentration of copper(II) sulfate in the sample. Be sure your answer has the correct number of significant digits.arrow_forward
- (c) Cl2(g) + KBr(aq) → KCI(aq) + Br2(g) O combination O decomposition O displacement molecular equation Cl2(g) + reducing agent KBr(aq) KCI(aq) + Br2(g) oxidizing agent total ionic equation | Cl2(g) + CI (aq) + net ionic equation K*(aq) + Br (aq) K+(aq) + Br2(g) Cl2(g) + K+(aq) + Br (aq) → K+(aq) + CI (aq) + | Br2(g)arrow_forwardChemists working with fluorine and its compounds some- times find it helpful to think in terms of acid-base reac- tions in which the fluoride ion (F¯) is donated and ассеpted. (a) Would the acid in this system be the fluoride donor or fluoride acceptor? (b) Identify the acid and base in each of these reactions: CIF;O2 + BF; CIF,O, · BF, -- TiF, + 2 KF – K2[TiF,]arrow_forward7B.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY