
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please correct answer and don't use hand rating
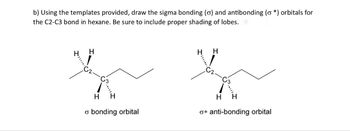
Transcribed Image Text:b) Using the templates provided, draw the sigma bonding (o) and antibonding (o*) orbitals for
the C2-C3 bond in hexane. Be sure to include proper shading of lobes.
H
H
H
H
H
H
H
σ bonding orbital
o* anti-bonding orbital
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- The actual structure of SF4 places the lone pair in only one of two possible sites. To discuss the stability of the observed structures, we need only consider bond angles LP/LP ii. BP/BP >> LP/BP > LP/LP LP/BP > BP/BP >> LP/LP LP/LP >>> LP/BP > BP/BP LP/LP = LP/BP = BP/BP ii. iv. V.arrow_forwardPls help ASAP.arrow_forwarda) Draw Lewis structures for each of the compounds below. Be sure to show all bonding and non-bonding valence electrons. Also, clearly indicate any formal charges on atoms. b) Consider the molecular geometry information given for each compound below: based on this information, specify the orbitals that each atom could use in σ- and à-bonding (sp², sp³, p, etc.) and for holding non-bonding electron pairs. Explain how your orbital assignments are consistent with the observed geometries. H₂CO (formaldehyde) HCO-angle 120⁰ CH3CO₂ (acetate anion) HCC-angle ≈ 109⁰ CCO-angle OCO≈ 120° DCH=CHBr(1-bromo-2-deuterioethylene) (two isomers) (all atoms are coplanar) (CH3)2SO (dimethylsulfoxide, DMSO) HCS-angle CSO CSC≈ 109⁰ ≈arrow_forward
- Let us construct the molecular orbital diagram of ethylene (in pieces). a. First, construct the MO diagram of linear carbene (CH2). Draw pictures of all 6 orbitals b. Now bend the carbene to a bond angle of about 120°. How does this change your MO diagram? Draw pictures of all 6 orbitals. c. Now bring two of these carbene molecules together to make ethylene. Draw pictures of all 12 orbitals.arrow_forwardSee image belowarrow_forwardB PLEASEarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning