
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Part 1: Which of the following compounds exhibits the WEAKEST intermolecular forces? Select the single best answer.
Part 2: Which of the following compounds exhibits the STRONGEST intermolecular forces? Select the single best answer.
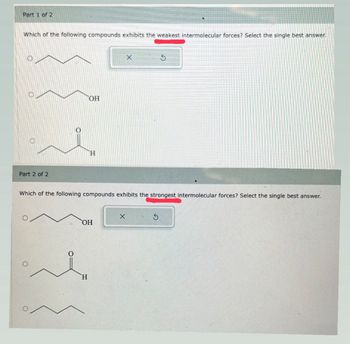
Transcribed Image Text:Part 1 of 2
•
Which of the following compounds exhibits the weakest intermolecular forces? Select the single best answer.
Part 2 of 2
OH
H
x
5
Which of the following compounds exhibits the strongest intermolecular forces? Select the single best answer.
OH
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- What are intermolecular forces? How do they differ from intramolecular forces? What are dipole-dipole forces? How do typical dipole-dipole forces differ from hydrogen bonding interactions? In what ways are they similar? What are London dispersion forces? How do typical London dispersion forces differ from dipole-dipole forces? In what ways are they similar? Describe the relationship between molecular size and strength of London dispersion forces. Place the major types of intermolecular forces in order of increasing strength. Is there some overlap? That is, can the strongest London dispersion forces be greater than some dipole-dipole forces? Give an example of such an instance.arrow_forwardClassify each of the following statements as true or false. a Intermolecular attractions are stronger in liquids than in gases. b Substances with weak intermolecular attractions generally have low vapor pressures. c Liquids with high molar heats of vaporization usually are more viscous than liquids with low molar heats of vaporization. d A substance with a relatively high surface tension usually has a very low boiling point. e All other things being equal, hydrogen bonds are weaker than induced dipole or dipole forces. f Induced dipole forces become very strong between large molecules. g Other things being equal, nonpolar molecules have stronger intermolecular attractions than polar molecules. h The essential feature of a dynamic equilibrium is that the rates of opposing changes are equal. i Equilibrium vapor pressure depends on the concentration of a vapor above its own liquid. j The heat of vaporization is equal to the heat of fusion, but with opposite sign. k The boiling point of a liquid is a fixed property of the liquid. l If you break shatter an amorphous solid, it will break in straight lines, but if you break a crystalline solid, it will break in curved lines. m Ionic crystals are seldom soluble in water. n Molecular crystals are nearly always soluble in water. o The numerical value of heat of vaporization is always larger than the numerical value of heat of condensation. p The units of heat of fusion are kJ/gC. q The temperature of water drops while it is freezing. r Specific heat is conerned with a change in temperature.arrow_forwardFor liquid-state samples of the following diatomic molecules, indicate the type or types of intermolecular forces (dipoledipole interactions, hydrogen bonding, London forces) present. There may be more than one correct answer in a given situation. a. H2 b. HF c. CO d. F2arrow_forward
- Indicate whether or not each of the following statements about intermolecular forces is true or false. a. Hydrogen bonds are extra strong London forces. b. A London force is a very weak permanent dipoledipole interaction. c. The strength of dipoledipole interactions increases as molecular polarity increases. d. All molecules with H atoms can participate in hydrogen bonding.arrow_forwardThe compounds ethanol (C2H5OH) and dimethyl ether (CH3OCH3) have the same molecular formula. Which is expected to have the higher surface tension? Why?arrow_forwardWhich member of each of the following pairs of compounds has the higher boiling point? (a) O2 or N2 (b) SO2 or CO2 (c) HF or HI (d) SiH4 or GeH4arrow_forward
- You and a friend each synthesize a compound with the formula XeCI2F2. Your compound is a liquid and your friend's compound is a gas (at the same conditions of temperature and pressure). Explain how the two compounds with the same formulas can exist in different phases at the same conditions of pressure and temperature.arrow_forwardLiquid methanol, CH3OH, is placed in a glass tube. Is the meniscus of the liquid concave or convex? Explain briefly.arrow_forwardWhat types of solids are these substances? (a) The hydrocarbon decane, C10H22, has a melting point of 31 C and is a poor electrical conductor. (b) Solid MgCl2 has a melting point of 714 C and conducts electricity only when melted.arrow_forward
- Which of the following substances can be liquefied by applying pressure at 25C? For those that cannot, describe the conditions under which they can be liquefied. Substance Critical Temperature Critical Pressure Sulfur dioxide, SO2 158C 78 atm Acetylene, C2H2 36C 62 atm Methane. CH4 82C 46 atm Carbon monoxide, CO 140C 35 atmarrow_forwardIndicate whether each of the following statements concerning boiling and boiling point is true or false. a. A liquid can be made to boil at temperatures higher than its normal boiling point. b. A liquid can be made to boil at temperatures lower than its normal boiling point. c. In a boiling liquid, vapor formation occurs within the body of the liquid. d. To compare the boiling points of two different liquids, the external pressure should be the same.arrow_forwardDefine the following terms and describe how each depends on the strength of the intermolecular forces. a. surface tension b. viscosity c. melting point d. boiling point e. vapor pressurearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning