
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
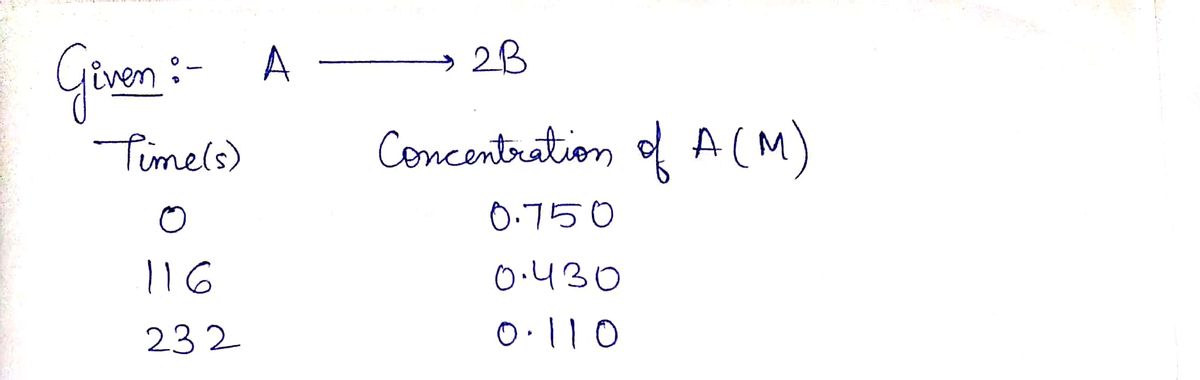
Determine the average rate of change of B from ?=0 s to ?=232 s.
A⟶2B
| Time (s) | Concentration of A (M) |
|---|---|
| 0 | 0.750 |
| 116 | 0.430 |
| 232 | 0.110 |
Expert Solution
arrow_forward
Step 1
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6) The decomposition of reactant R was studied, and the data given below was obtained at 100.0 °C: R> products time (min) concentration (M) 0.120 35.6 5.83 17.8 25.6 0.0910 0.060 0.046 0.030 a) What is the half-life of the reaction in minutes? Explain. b) Various plots of the concentration of the reactant versus time are given below. What is the order of the reaction? Explain. ILD is 20 time (min) time (min) 05 -35 time (min) c) What is the value of the rate constant at 100.0 °C? d) Would you expect the half-life of the reaction to be longer, shorter, or the same at 60.0 °C? Explain. e) The activation energy in the forward direction is 95.0 kl/mol, and the enthalpy change for the forward reaction is 30.0 kl/mol. Sketch the reaction coordinate diagram for this reaction. ERER ARarrow_forwardPlease complete answers 30 mint asaparrow_forwardWhat is the half life of the following reaction, given the following set of conditions (Hint: observe the units for the rate constant to determine the order of the reaction!): 2 HI (g) → H2 (g) + I2 (g) Initial concentration of HI = 1.00 M k = 1.2 x 10-3 M-1 s-1 Select one: a. 420 s b. 240 s c. 580 s d. 830 sarrow_forward
- Correct answers are given in second picture!!! Please show me how to get them and explain!arrow_forward(35) The following data were obtained for the hypothetical reaction 2A + B → products. [A]o (M) [B]o (M) Initial Rate (M/s) 0.2 0.1 0.2 0.2 20 0.6 0.1 15 What is the overall order of this reaction? а. 4 b. ½ с. 0 d. 3 е. 1arrow_forwardDetermine the average rate of change of B from t = 0 s to = 362 s. A → 2B rate = M/s Time (s) 0 181 362 Concentration of A (M) 0.720 0.425 0.130arrow_forward
- 43-46 Consider the time and concentration data for: 2 NO2(g) 2 NO(g) + O2(g). Time, s [NO2] 43 a. How long is the first half-life? Write the value and units 0.100 0.072 20 b. How long is the second half-life? Write the value and units 0.050 50 44. Write the rate law for this reaction. Explain why you made this choice. 0.036 90 0.025 150 45. What is the value of the rate constant? Write the general equation, the calculation equation with units, and the answer with units 46. How much time is needed until only 0.010 M NO2 remains? Write the general equation, the calculation with units, and the answer with units carrow_forwardDetermine the average rate of change of B from t = 0 s to t = 282 s. A2B rateB = M/s Time (s) 0 141 282 Concentration of A (M) 0.610 0.380 0.150arrow_forwardDoubling the concentration of NO at constant temp is expected to: a.) increase rate by factor of 8 b.) increase rate by factor of 2 c.) doesn't change rate d.) increase rate by factor of 4arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY