
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Doubling the concentration of NO at constant temp is expected to:
a.) increase rate by factor of 8
b.) increase rate by factor of 2
c.) doesn't change rate
d.) increase rate by factor of 4
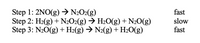
Transcribed Image Text:Step 1: 2NO(g) → N2O2(g)
Step 2: H2(g) + N2O2(g) → H2O(g) + N20(g)
Step 3: N20(g) +H2(g) → N2(g) + H2O(g)
fast
slow
fast
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How did the rate change when the reaction was performed at a higher temperature? why? expalin at the molecular practice level.arrow_forwardNonearrow_forwardQuestion: Relationship to reaction profiles: catalyst / enzymes lower activation energy Help please it better explain or example or links to better explain and understandarrow_forward
- Question 15 Consider the reaction of methyl chloride (CH3CI) with NaN3 (nucleophile). What is the effect of doubling the concentration of NaNg on the rate of the reaction? a) Rate remains same b) Rate increases by a factor of 2 c) Rate increases by a factor of 4 d) Cannot be determined a d. b.arrow_forwardUse the following data table to answer the following questions. Experiment Concn. of A (M) Concn. of B (M) Initial rate (M/s) 1 0.10 0.010 1.2e-3 2 0.10 0.040 4.8e-3 3 0.20 0.010 2.4e-3 d. The rate constant of the reaction is e. The rate of reaction when concentration of A and B are 0.15M and 0.075M isarrow_forward/::://::(((((arrow_forward
- What is true about the diagram? A.) The step from A->B is the slow step because activation energy is high. B.) Step B-->C is the rate limiting step. C.) The reaction is endothermic. D.) There is one transition state showing. Please refer to the attached photo for the diagram.arrow_forwardA reactant is found to have an order of zero in a chemical reaction. What would be the effect on the rate if the concentration of that reactant were decreased by half? options: The rate would quadruple The concentration has no effect on the rate The rate would decrease by half The rate would doublearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY