
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
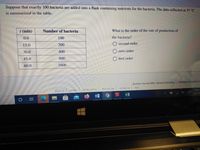
Transcribed Image Text:Suppose that exactly 100 bacteria are added into a flask containing nutrients for the bacteria. The data collected at 37 °C
is summarized in the table.
t (min)
Number of bacteria
What is the order of the rate of production of
0.0
100
the bacteria?
15.0
200
O second order
30.0
400
zero order
45.0
800
first order
60.0
1600
Question Source: MRG - General Chemistry, Publisher: University Sc
terms of use
contact us
help
about us
careers
privacy policy
10:55
2/11/2
近
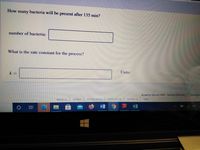
Transcribed Image Text:How
bacteria will be present after 135 min?
many
number of bacteria:
What is the rate constant for the process?
Units:
k =
Question Source: MRG - General Chemistry, Publisher:
privacy po icy
terms of use
contact us
help
about us
careers
四 X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A certain reaction is first order in N, and second order in H,. Use this information to complete the table below. Round each of your answers to 3 significant digits. [N] [1] initial rate of reaction 1.50 M 2.34 M 77.0 M/s O M/s 2.12 M 2.34 M O M/s 0.300 M 11.7 Marrow_forwardA certain reaction is second order in N, and second order in H,. Use this information to complete the table below. Round each of your answers to 3 significant digits. [N-] initial rate of reaction 0.153 M 1.80 M 13.0 M/s x 5 ? 0.245 M 1.80 M O MIs 0.618 M 0.445 M M/sarrow_forwardDetermine the average rate of change of B from t = 0 s to = 362 s. A → 2B rate = M/s Time (s) 0 181 362 Concentration of A (M) 0.720 0.425 0.130arrow_forward
- A certain reaction is second order in N, and second order in H,. Use this information to complete the table below. Be sure each of your answer entries has the correct number of significant digits. [N] [4] Initial rate of reaction 2.41 M 0.733 M 19.0 M/s 0.501 M 0.733 M Mls 0.873 M 2.02 M Mls dlo Ararrow_forwardThe decomposition of a chemical follows the Zero order with a rate constant, k = 0.00543 M/s. If the initial concentration of the reactant is 2.32. what is the concentration after 900 seconds?. The decomposition of a chemical is in the second order with a rate constant, k = .0123 M-1min-1. If the initial concentration of the reactant is 0.765 M. What is the concentration after 15 minutes.arrow_forward1. At elevated temperatures, nitrous oxide decomposes according to the equation2N2O(g)⟶2N2(g)+O2(g) Time (min) 0 60 90 300 600 [N2O] 0.250 0.228 0.216 0.128 0.0630 Given the following data, determine whether the reaction is zeroth, first, or second order. a) first b) second c) zerotharrow_forward
- 3) The reaction 2NO(g) + O2(g) → 2 NO₂(g) was studied, and the following data were obtained where Δ[02] At Rate = [NO]. [0₂]0 (molecules/cm³) (molecules/cm³) 1.00 x 1018 3.00 x 10¹8 2.50 × 1018 1.00 x 1018 1.00 × 10¹8 2.50 × 1018 Submit Answer Initial Rate (molecules/cm³.s) 2.00 × 1016 1.80 x 10¹7 3.13 x 10¹7 What would be the initial rate for an experiment where [NO], = 1.57 x 10¹8 molecules/cm³ and [0₂]0 = 8.82 x 10¹8 molecules/cm³? 3 Rate = molecules/cm³.s Try Another Version [References] 5 item attempts remaining Show Hintarrow_forward17arrow_forwardA certain reaction is second order in N₂ and first order in H₂. Use this information to complete the table below. Round each of your answers to 3 significant digits. 1.46 M 0.653 M 1.57 M F 2.72 M 0.653 M 0.351 M initial rate of reaction. 33.0 M/s M/S M/S X Sarrow_forward
- Save An Sugar reacts with water in acidic condition to give glucose and fructose. The reaction is first order in sugar. At 25 °C, it takes 3.33 h for the concentration of sugar to drop to ½ of its original concentration. How many hours are required for the concentration to drop from 0.800 M to 0.0500 M? O 9.23 h 6.93 h 13.3 h O4.80 h 53.3 h A Moving to another question will save this response. Question 1 of 25 >> Close Window Esc F1 F2 F3 F4 F5 F6 F7 PrtSc Insert Delete F8 F9 F10 F11 F12 %23 $4 & 1 2 4 8. Backspace Q W T. Y | P ab D F G H. J K 11 Z V + I/ V - MI INarrow_forwardSuppose that exactly 100 bacteria are added into a flask containing nutrients for the bacteria. The data collected at 37 °C is summarized in the table. t (min) 0.0 19.0 38.0 57.0 76.0 How many bacteria will be present after 133 min? number of bacteria: Number of bacteria 100 200 400 800 1600 k= What is the rate constant for the process? What is the order of the rate of production of the bacteria? zero order first order second order Units:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY