
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:NAMI
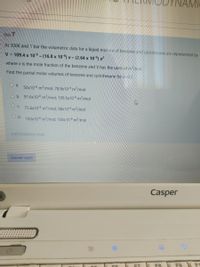
Sbru 7
At 300K and 1 bar the volumetric data for a liquid mixture of benzene anid cyclohexane are represented by
V = 109.4 x 106 - (16.8 x 10 6) x- (2.64 x 106) x?
where x is the mole fraction of the benzene and V has the units of m/mol.
Find the partial molar volumes of benzene and cyclohexane for x-0.2
Oa.
50x10-6 m³/mol; 78.9x10-6 m³/mol
O b. 91.6x10-€ m³/mol; 109.5x10-6 m³/mol
Oc.
75.6x10-6 m³/mol; 98x10-6 m³/mol
100x10-6 m³/mol; 108x10-6 m³/mol
210707090303-4705
Önceki sayfa
Casper
F8
F9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 1.9 Using van der Waals equation, estimate the pressure required to compress two grams moles of nitrogen into a volume of 3,000 cm at 20°C.arrow_forwardAir is generally assumed to be 79% nitrogen and 21% oxygen on a volume basis. What is the composition of air on a mass basis?arrow_forward3. Pressure and entropy of degenerate Fermi gas. (a) Show that a Fermi electron gas in the ground state exerts a pressure (3n²)2/3 h? (N\S/3 N\5/3 (90) 5 m V In a uniform decrease of the volume of a cube every orbital has its energy raised: The energy of an orbital is proportional to 1/L? or to 1/V213. (b) Find an expression for the entropy of a Fermi electron gas in the region z « Ep. Notice that o → 0 as t – 0.arrow_forward
- A liquid mixture is made of 15 kg Benzene (MW= 78), 30 kg Toluene (MW=92) and 15 kg Xylene (MW=106) the average molecular weight of this mixture is: Answer:arrow_forwardOxygen is contained in a cylinder at a pressure of 95 bar and a temperature of 185.52 K. Estimate the molar volume in units ?^3/??? and the specific volume in units ?^3/?? using : (a) The ideal gas equation of state (b) Van der Waals equation of state (c) Redlich-Kwong equation of state (d) Lee Kesler correlation Argue why oxygen is a real gas under these conditions. Evaluate the accuracy of each correlation.arrow_forwardQUESTION 1 Calculate the pressure exerted by Ar for a molar volume 0.39L at 265K using the van der Waals equation of state. The van der Waals parameters a and b for Ar are 1.355 bar dm6 mol-2 and 0.0320 dm³mol-1, respectively. Please write your answer (unit: bar) round to 1 decimal places, as 12.2. Please do not add unit to your answer.arrow_forward
- (a)Using the compressibility chart, determine the compression factor (Z). Express your final answers in 3 significant figures (b)What would be the corresponding state of carbon dioxide? (Pc = 72.9 atm, Tc = 304.2 K) T (K) 200 P (atm) Tс (К) Pc (atm) Gas Argon 23.0 150.7 48.0 1.1 1.0 T- 2.00 0.9 I=1.50 0.8 Tx =1.30 0.7 to 0.6 T=1.20 0.5 T-1.10 0.4 Legend: X Methane O Ethylene A Ethane O Propane O n-Butane Iso-pentane • n-Heptane A Nitrogen T =1.00 0.3 Carbon dioxide Water 0.2 Average curve based on data on hydrocarbons 0.1 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0 Reduced pressure P x of xarrow_forwardPHYSICAL CHEMISTRY:Calculate the pressure exerted by 1.0 mol C2H6 at 1000 K in 100 cm3 using (a) the ideal gas equation and (b) the van der Waals equation. The van der Waals constant for C2H6 are: a = 5.562 bar dm6 mol-2 ; b = 0.06380 dm3 mol-1 answer:[831; 1.72 x 10-3]arrow_forwardConsider cyclohexane at 175ºC. Determine Z and V (cm^3/mol) when P = 8.74 bar (saturated liquid)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The