
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
answer is wrong need help

Transcribed Image Text:QUESTION 2
Estimate the molar Gibbs energy change (unit: J/mol) of water when the pressure acting on it is increased from 100 kPa to
345 kPa. Density of water is 1g/cm³.
Instruction: Please enter your result with 2 decimal places. For example: 12.669 is written as 12.70

Transcribed Image Text:QUESTION 2
Estimate the molar Gibbs energy change (unit: J/mol) of water when the pressure acting on it is increased from 100 kPa to
345 kPa. Density of water is 1g/cm³.
Instruction: Please enter your result with 2 decimal places. For example: 12.669 is written as 12.70
Expert Solution
arrow_forward
Equation for the Isothermal Molar Gibbs Free Energy change.
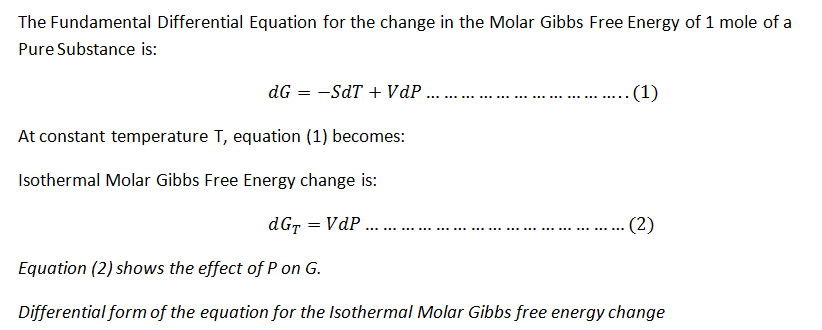
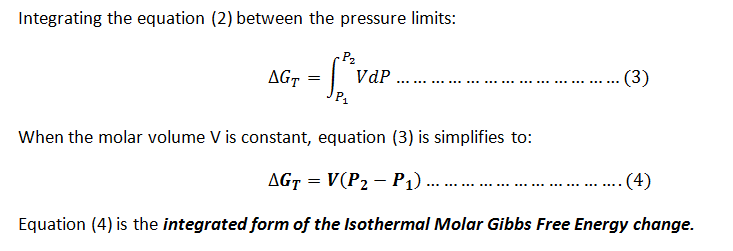
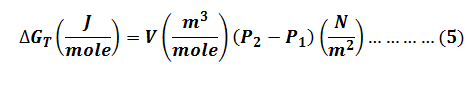
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Similar questions
- 6. Draw the following point coordinates: a. [110] b. [1 % 1] c. [0 1 2]arrow_forwardlist as many factors as you can think of that will affect a house's heating/cooling " needs. Conduction Convection Other factorsarrow_forwardH(a9) + F Cap) K-3.5x10* Find all []ó's (Jo O.200M change [ Jearrow_forward
- I need help with molarity of NaOHarrow_forwardDiagrams need also for this. 2-Electrolyte: The intricate dance of electrons is aided by a liquid bridge filled with a redox mediator between the anode and counter electrode.arrow_forwardFor which set of crystallographic planes will a first-order diffraction peak occur at a diffraction angle of 44.53° for FCC nickel (Ni) when monochromatic radiation having a wavelength of 0.1542 nm is used? The atomic radius for Ni is 0.1246 nm. 1) ( eTextbook and Media Assistance Usedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The