
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
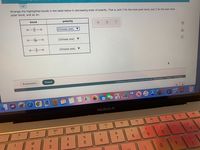
Transcribed Image Text:Arrange the highlighted bonds in the table below in decreasing order of polarity. That is, pick 1 for the most polar bond, pick 2 for the next most
polar bond, and so on.
bond
polarity
H-ö-H
(Choose one)
H-Se-H
(Choose one) ▼
do
(Choose one)
Explanation
Check
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use I Privacy | Accessibility
Aa
13
MacBook Air
4)
DD
88
F9
F8
F4
F5
F3
F1
&
#3
2$
9.
6.
7
8.
3
4
又:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw a Lewis structure for each compound and fill in the missing information. Name of Molecular Shape Formula Process Work Lewis Structure Type of Electron Pairs VSEPR Notation Polarity of Molecule CH2C12|arrow_forwardOrganic Chemistry HW see image attached Please illustrate on the given image the correct answers - not handwritten Thank youarrow_forwardDecide whether the Lewis structure proposed for each molecule is reasonable or not. molecule proposed Lewis structure BF3 SF 3 CO₂ :F: | -B-F: :F- .. | SIF: S-F: || .. :F: 01010 Is this a reasonable structure? If not, why not? OYes, it's a reasonable structure. No, the total number of valence electrons is wrong. The correct number is: O No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: Yes, it's a reasonable structure. O No, the total number of valence electrons is wrong. The correct number is: O No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: 0 Yes, it's a reasonable structure. No, the total number of valence electrons is wrong. The correct number is: No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: * If two or more atoms have the wrong number of valence electrons around them, just enter the chemical symbol for the atom as many…arrow_forward
- Decide whether the Lewis structure proposed for each molecule is reasonable or not. molecule proposed Lewis structure OF 2 BeH₂ PF4 || :O: H - Be H F - P F W ... :F: :F: Is this a reasonable structure? If not, why not? Yes, it's a reasonable structure. O No, the total number of valence electrons is wrong. The correct number is: No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: O Yes, it's a reasonable structure. No, the total number of valence electrons is wrong. The correct number is: O No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: O Yes, it's a reasonable structure. No, the total number of valence electrons is wrong. The correct number is: No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: 0 * If two or more atoms have the wrong number of valence electrons around them, just enter the chemical symbol for the atom as many times as…arrow_forwardFor each bond, show the direction of polarity by selecting the correct partial charges.arrow_forwardEvery atom in the molecule below shows a correct bonding pattern (free electron pairs are not shown). The molecule also contains at least 2 Cs and at bond: // H-C-C | ОН O True O False エーOーエarrow_forward
- Name the highlighted chemical group in each molecule. Lewis structure H H H-C-C=0 H-C-H H H-Ö: | H-C-H | H H H H-C- -- | | H H - G name of highlighted group பarrow_forwardDecide whether the Lewis structure proposed for each molecule is reasonable or not. proposed Lewis structure Is this a reasonable structure? If not, why not? molecule O Yes, it's a reasonable structure. O No, the total number of valence electrons is wrong. NH, H N=H The correct number is:| No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: LI Yes, it's a reasonable structure. :F: O No, the total number of valence electrons is wrong. BF, The correct number is: :F-B –F: O No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: U Yes, it's a reasonable structure. F No, the total number of valence electrons is wrong. The correct number is: IF No, some atoms have the wrong number of electrons around them. .. F: The symbols of the problem atoms are: U * If two or more atoms have the wrong number of valence electrons around them, just enter the chemical symbol for the atom as many times as…arrow_forwardEthyl acetate, C4H8O2, is a fragrant substance used both as a solvent and as an aroma enhancer. Its Lewis structure is (Figure 1). Figure 1 of 1 H O: H H H-C-C-0-C-C-H нн Type here to searcharrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY