
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
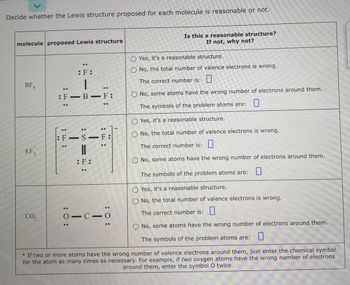
Transcribed Image Text:Decide whether the Lewis structure proposed for each molecule is reasonable or not.
molecule proposed Lewis structure
BF3
SF 3
CO₂
:F:
|
-B-F:
:F-
..
|
SIF:
S-F:
||
..
:F:
01010
Is this a reasonable structure?
If not, why not?
OYes, it's a reasonable structure.
No, the total number of valence electrons is wrong.
The correct number is:
O No, some atoms have the wrong number of electrons around them.
The symbols of the problem atoms are:
Yes, it's a reasonable structure.
O No, the total number of valence electrons is wrong.
The correct number is:
O No, some atoms have the wrong number of electrons around them.
The symbols of the problem atoms are: 0
Yes, it's a reasonable structure.
No, the total number of valence electrons is wrong.
The correct number is:
No, some atoms have the wrong number of electrons around them.
The symbols of the problem atoms are:
* If two or more atoms have the wrong number of valence electrons around them, just enter the chemical symbol
for the atom as many times as necessary. For example, if two oxygen atoms have the wrong number of electrons
around them, enter the symbol O twice.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 19 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. Write Lewis structures for the following. Show ALL resonance structures whereapplicable. For a, the central atom is carbon: a. SCN- b. N3- 2. Benzene (C6H6) consists of a six membered ring of carbon atoms with one hydrogen bonded to each carbon. Write Lewis structures for benzene, including resonance structures.arrow_forwardO ELECTRONIC STRUCTURE AND CHEMICA... Deciding whether a Lewis... Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure [O=C-H]* :0: : CIC CI: [¤¤-6: 0/5 Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: 0 No, it has the right number of valence electrons but doesn't satisfy the octet rule. 000 The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: 0 Alia V No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* X Ar If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example,…arrow_forward3. Draw the Lewis structure of carbon dioxide below. Then next to each atom in the molecule, write whether it has a partial positive or partial negative charge using the d' and & symbols. Finally, calculate the AEN for the C-O bondarrow_forward
- Determine whether a molecule will have one or more resonance structures. Guiacol is a molecule responsible for a vanilla aroma. Will this molecule have resonance structures? Explain. A structural formula is provided here: C6H4(OH)(OCH3) and a structure is shown below. H. H H.arrow_forwardhow do i fill in the chart whats the answerarrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure esc H= Br ! : 0: 1 Cl Explanation Q A :0: || g N The symbols of the problem atoms are:* 0 * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "O,O". : 0: 2 Cl: Check W S Yes. O No, it has the wrong number of valence electrons. The correct number is: O No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* 0 O Yes. O No, it has the wrong number of valence electrons. The correct number is: O No, it has the right number of valence electrons but doesn't satisfy the octet rule. 0 Is the proposed Lewis structure reasonable? The symbols of the problem atoms are:* Yes. O No, it has the wrong number of valence electrons. The correct number is: 0 No, it has the right number of valence electrons…arrow_forward
- Select only, and all, of the drawings, shown below, which are acceptable Lewis diagrams for the species indicated. : :J: :CI: :CI: =C-H :CI: : CI: H Lewis structure of POCI3? Lewis structure of SO₂Cl₂? Lewis structure of NOCI? Lewis structure of HCN? Lewis structure of CHCI3?arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? ○ Yes. : 0: H- =0 =0 : C : 0: No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* O Yes. O No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* O Yes. O No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "O,O". X Garrow_forward= O ELECTRONIC STRUCTURE AND CHEMICAL BONDING Counting bonding and nonbonding electron pairs in a Lewis... Below is the Lewis structure of the methoxide H H:C:O: H e (CH₂O) ion. Count the number of bonding pairs and the number of lone pairs around the carbon atom. bonding pairs: lone pairs: X Ś 1/5arrow_forward
- Look at the molecule depicted below, several atoms need more or less electrons to complete its octet. Select all that apply from the list below. :F: H3C-Ċ- | | : F :0 -H The O with a double bond needs two electrons. the C with a double bond needs to lose two electrons. The H needs two electrons. The O with two single bonds needs two electrons. F needs two more electrons. O The C with four bonds needs two electrons. :o:arrow_forwardDecide whether the Lewis structure proposed for each molecule is reasonable or not. proposed Lewis structure Is this a reasonable structure? If not, why not? molecule O Yes, it's a reasonable structure. O No, the total number of valence electrons is wrong. NH, H N=H The correct number is:| No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: LI Yes, it's a reasonable structure. :F: O No, the total number of valence electrons is wrong. BF, The correct number is: :F-B –F: O No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: U Yes, it's a reasonable structure. F No, the total number of valence electrons is wrong. The correct number is: IF No, some atoms have the wrong number of electrons around them. .. F: The symbols of the problem atoms are: U * If two or more atoms have the wrong number of valence electrons around them, just enter the chemical symbol for the atom as many times as…arrow_forwardO A student proposes the following Lewis structure for the carbon dioxide (CO₂) molecule. osoft C W W Microsoft :01C=0: Assign a formal charge to each atom in the student's Lewis structure. Microsoft 6.52.210... esc atom left O C right O Explanation 1 Q A formal charge 2 U U U Check 8,999 W S 280 Q # 3 E X 17 D DEC 8 4 5 R do LO % bartleby 5 LL tv ♫ NIN T 6 G Y & 2022 McGraw Hill L 7 H * 00 U 8 LEarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY