
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Will you mind to help me with this question, please?
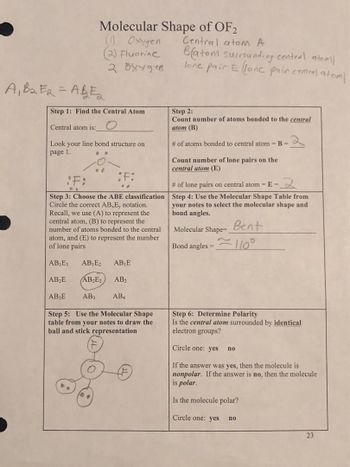
Transcribed Image Text:A₁B₂E2=AB=2
Molecular Shape of OF2
Step 1: Find the Central Atom
Central atom is:
Look your line bond structure on
page 1.
(1) Oxygen
(2) Fluorine
2 oxygen
¡F:
Step 3: Choose the ABE classification
Circle the correct AB E, notation.
Recall, we use (A) to represent the
central atom, (B) to represent the
number of atoms bonded to the central
atom, and (E) to represent the number
of lone pairs
AB₁E3
AB₁E₂ AB₁E
AB₂E₂
AB2
AB3
AB4
Step 5: Use the Molecular Shape
table from your notes to draw the
ball and stick representation
AB₂E
AB E
Central atom A
Batom surrounding central atom/
lone pair E llone pair central atom/
Step 2:
Count number of atoms bonded to the central
atom (B)
# of atoms bonded to central atom = B =
Count number of lone pairs on the
central atom (E)
# of lone pairs on central atom=E=
2
Step 4: Use the Molecular Shape Table from
your notes to select the molecular shape and
bond angles.
Bent
Molecular Shape=
Bond angles=110°
Step 6: Determine Polarity
Is the central atom surrounded by identical
electron groups?
Circle one: yes
If the answer was yes, then the molecule is
nonpolar. If the answer is no, then the molecule
is polar.
Is the molecule polar?
Circle one: yes
no
no
23
Expert Solution
arrow_forward
Step 1
In the question we have asked to tell whether molecule OF2 is polar or not, it's geometry, bond angle, ball and stick representation
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the reaction below to answer the following questions. HÇI HO H,0 HO a. The first nucleophile in the reaction is b. The catalyst in the reaction is c. The newly created functional group in the reaction is called a(n) d. The first step in the reaction mechanism is protonation of the group. e. The last step in the reaction mechanism is of the oxonium ion.arrow_forwardPlease fill in the tablearrow_forwardHelp with question 9arrow_forward
- write an equation for the reaction of methanol with sodium?arrow_forwardThe boiling points of various organic compounds are given below. Explain the reasons for the different be points. How do the functional groups of each compound effect their boiling points? 6. Boiling Point 141°C Compound | Propanoic acid 1-propanol 1-propanamine Propanal Propane 97°C 51°C 48°C -42°C bAdd hdrol 7. The compound shown is a common artificial sweetener, Circle and identify the functional groups in the molecule. Which functional groups can undergo hydrolysis? Draw structures to show the complete hydrolysis (either acidic or basic) of the compound. (Hint: hydrolysis may occur in more than one location.) anen HO. NH CH3 NH2 8. a) Valproic acid is an anti-seizure medication often administered in a sodium salt form. Draw out the reaction showing how the sodium salt of valproic acid is formed. Why is the salt form used? etoca) soslom lonoitanutlsgs at (woled pile bos aiolte n anuong le 8,9slicalyrtts mal or lonsis w bus bsCH, - CH, - CH oD mal e (biaE CH - CH, - CH, - CH,…arrow_forward454 | Chapter 15 Amines (a) Which of the two nitrogen atoms in epibatadine is 54 the stronger base? (b) Mark the three stereocenters in this molecule. Cl H N Narrow_forward
- 6. (Chapter 15-Q37) Indole is an aromatic heterocyclic that has a benzene ring fused to a pyrrole ring. Answer the following questions. Indole 6(a) What is the hybridization of N in this molecule? = 6(b) How many pi electrons N contributes to the ring? = 6() Which orbitals contribute to form a sigma bond between N and H in this molecule? = 6(c) What is the electronic relationship of Indole to naphthalene? Give the answer by comparing number of rings and number of pi electrons in both compounds, write x rings, y pi electrons=|arrow_forward(A) Molecule A Molecule B Molecule C Molecule D Molecule E (B) Molecule F (E) Of the molecules depicted above, which two could be the products of an esterification reaction, which is a type of condensation reaction? OH 'OH (C) (D)arrow_forwardConsider the proton transfer reaction between the following compounds. + لا F F OH 12 Farrow_forward
- 2. What type of chemical reaction is illustrated by the following reaction? CH3OOCH3 + H2O ⎯→ CH3OOH + CH3OH a. oxidation/reduction b. substitution c. neutralization d. hydrolysis e. dehydration synthesis (condensation)arrow_forwardName the product of this reaction. Cl H H H—C—C—CH H H H base ?arrow_forwardPart B Write the balanced chemical equation for the dissociation of a-hydroxypropanoic acid in water. Express your answer as a chemical equation, using condensed formulas to represent the compounds in the equation. ΑΣΦ ? C₂ H₂ (OH)COOH + H₂O=C₂ H₂ (OH)COO¯ + H₂O+ A chemical reaction does not occur for this question. Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remainingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY