
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
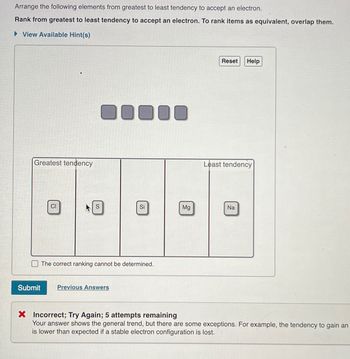
Transcribed Image Text:Arrange the following elements from greatest to least tendency to accept an electron.
Rank from greatest to least tendency to accept an electron. To rank items as equivalent, overlap them.
► View Available Hint(s)
Greatest tendency
Submit
00000
Si
The correct ranking cannot be determined.
Previous Answers
Mg
Reset
Help
Least tendency
Na
X Incorrect; Try Again; 5 attempts remaining
Your answer shows the general trend, but there are some exceptions. For example, the tendency to gain an
is lower than expected if stable electron configuration is lost.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The element in which the 5th energy is first completely filled is not yet known. O True Falsearrow_forwardpreform a litmus tes for the following elements Na Karrow_forward69°F Mostly cloudy Re-order each list in the table below, if necessary, so that the atoms or ions in it are listed in order of decreasing size. atoms or ions 2+ Be, BB F.F, Ne F, C1, C1 Explanation atoms or ions in order of decreasing size 0.0.0 0.0.0 0.0.0 Check ▬▬▬ Search X 00 S 99+ 4 Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Priarrow_forward
- Fill in the orbital energy diagram for the carbon atom. E 2s 1s Submit Answer Retry Entire Group 9 more group attempts remaining Previous Next Email Instructor Save and Exit Cengage Learning | Cengage Technical Supportarrow_forwardAn atomic anion with a charge of -1 has the following electron configuration: 15²2s²2p 69°F Mostly cloudy esc What is the chemical symbol for the ion? How many electrons does the ion have? How many 1s electrons are in the ion? ? Explanation (@ f2 * Check # 3 * f4 $ 4 U لا 0 O Search % 40 C X D 4- 17 +▷ 8. fa 2022arrow_forwardRe-order each list in the table below, if necessary, so that the atoms or ions in it are listed in order of decreasing size. atoms or ions 2- Se, Se, Br F, CI, CI S, Al, P Explanation atoms or ions in order of decreasing size 0.0.0 0.0.0 0.0.0 Check X 00 G Ⓒ2022 MCGIDw Mill LLC. All Rights Reserved. Terms of Usearrow_forward
- me File Edit View History Bookmarks People Tab Window Help HCc Dashboar x E Buy Essay x 5% O G find some x © Periodic A ALEKS - J X HUc Chapter 5 x G convert C A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQİHQRDYV_6Ux63SypJXz0Coxvwqgg4JkWI74Meq7fCi1Pj.. O GASES Search Calculating partial pressure in a gas mixture S jacquehr mail.goo Jacqueline Profile - web.robl Cuteding of the mi A 9.00 L tank at 3.36 °C is filled with 13.2 g of boron trifluoride gas and 9.61 g of dinitrogen difluoride gas. You can assume both gases behave as ideal gases under these conditions. R Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits. Virtual E R - Roblox web.robl Virtual B group on mole fraction: dh boron trifluoride Manage appleid.a Your App partial pressure: | atm le for account mole fraction: dinitrogen diffluoride ble to partial pressure: ||atm Total pressure in tank: | atm ranscrip…arrow_forwardQuestion 4 of 21 View Policies Show Attempt History Current Attempt in Progress Your answer is partially correct. In PCIS, the Patom violates the octet rule by sharing 5 electrons. This is because the valence she elements in which n= 3, can hold a maximum of i electrons. 18 eTextbook and Media Save for Later Attempts: 1 of 2 used Submi P Type here to search a IDI W D F G Harrow_forwardarrow_forward
- Pls help ASAParrow_forwardSection:Chemistry 121 Team Instructor : Angela Herbert REPORT SHEET LAB Compounds and Their Bonds A. Ions: Transfer of Electrons Element Atom- | 1. Electron ic # 2. Electron- 3. Loss 4. Electron 5. Ionic 6.Symb 7. Name Configuration of Dot or Gain Configuration Charge ol of of Ion Atom Symbol of Ion Ion Sodium 11 1322p3 Na 1s252p Sodium lose 1 e 1* Na 1+ ion Nitrogen Aluminum Chlorine Calcium Охудen B. Ionic Compounds and Formulas Melting Point 801°C la Lilarrow_forwardNonearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY