
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Help
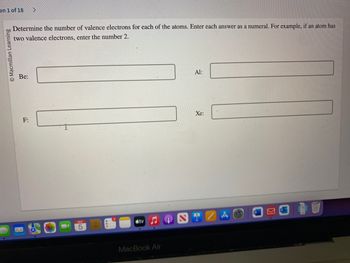
Transcribed Image Text:on 1 of 18
O Macmillan Learning
Determine the number of valence electrons for each of the atoms. Enter each answer as a numeral. For example, if an atom has
two valence electrons, enter the number 2.
Be:
D
F:
5
tv
MacBook Air
Al:
Xe:
29
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What are bus? How are ions formed from atoms? Do isolated atoms form ions spontaneously? To what do the termscationandanionrefer? In terms of subatomic particles, how is an ion related to the atom from which it is formed? Does the nucleus of an atom change when the atom is converted into an ion? How can the periodic table be used to predict what ion an element’s atoms will form?arrow_forwardFor the following ions, indicate whether electrons must begainedorlostfrom the parent neutral atom, andhow manyelectrons must be gained or lost. a.O2 c.Cr3+ e.Rb+ b.P3 d.Sn2+ f.Pb2+arrow_forwardWhich of the following is true about an individual atom? Explain. a. An individual atom should be considered to be a solid. b. An individual atom should be considered to be a liquid. c. An individual atom should be considered to be a gas. d. The state of the atom depends on which element it is. e. An individual atom cannot be considered to be a solid, liquid, or gas. Justify your choice, and for choices you did not pick, explain what is wrong with them.arrow_forward
- Reaction of 2.0 L of hydrogen gas with 1.0 L of oxygen gas yields 2.0 L of water vapor. All gases are at the same temperature and pressure. Show how these data support the idea that oxygen gas is a diatomic molecule. Must we consider hydrogen to be a diatomic molecule to explain these results?arrow_forward7.21 Theoretical models for the structure of atomic nuclei predict the existence of superheavy elements that have not yet been discovered and suggest that such elements might be fairly stable if they could be produced. So researchers are currently trying to synthesize these superheavy elements to test these theories. Element 117 has been synthesized and named Tennessine (Ts). What would the Lewis dot symbol be for this new element?arrow_forwardHow many valence electrons are needed to complete the outer valence shell of sulfur? a. 1 b. 2 c. 3 d. 4arrow_forward
- A combustion reaction involves the reaction of a substance with oxygen gas. The complete combustion of any hydrocarbon (binary compound of carbon and hydrogen) produces carbon dioxide and water as the only products. Octane is a hydrocarbon that is found in gasoline. Complete combustion of octane produces 8 L of carbon dioxide for every 9 L of water vapor (both measured at the same temperature and pressure). What is the ratio of carbon atoms to hydrogen atoms in a molecule of octane?arrow_forward2.85 Describe how the saying “opposites attract” corresponds with the mathematical representation of Coulomb’s law shown in Equation 2.1. Remember that attractive forces have negative values and repulsive forces have positive values.arrow_forwardpnc.com/women O Dashboa x Course X O The Wav x E CP grou X M Docume x E 3- Qual X G If you ha Xx course.html?courseld=16985674&OpenVellumHMAC=624ef941a8d 10e711a39b3488025ef9a#10001 v Correct S has 16 electrons. The noble gas that precedes S on the periodic table is neon, so the inner electron configuration is [Nel. Obtain the outer electron configuration by tracing the elements between Ne and S and assigning electrons to the appropriate orbitals. Because S is in row 3, as you trace across the row to S, which is in the third column of the p block, add two 3s electrons and three 3p electrons. As a result, the electron configuration for S is (Ne 3s 3p Part B Se Express your answer in condensed form in order of increasing orbital energy as a string without blank space between orbitals. For example, He 2s 2p? should be entered as [He]2s^22p^2. Submit Previous Answers Request Answer X Incorrect; Try Again; 2 attempts remaining Part C P Pearson Copyright © 2021 Pearson Education Inc. All…arrow_forward
- How did Thomson determine that the electrons have a negative charge?Drag the terms on the left to the appropriate blanks on the right to complete the sentences.arrow_forwardThe first five ionization energies (IE, through IE) of a Period 6 element have the following pattern: IIII IE₁ IE₂ IE3 IE4 IE5 Make a reasonable guess about which element this is. Enter its chemical symbol below. 0 X Ś alo 18 Ararrow_forwardCHEMISTRY (2021) ASSESSMENT CHEMISTRY I 12-3 (THOMAS WILLIAMS, ID: 12390719) Aluminum has three valence electrons, and oxygen has six valence electrons. What is the formula for aluminum oxide A AlgO3 Al,0 C AIO D Al203 Save Barrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning