
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
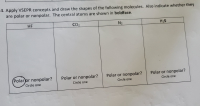
Transcribed Image Text:Apply VSEPR concepts and draw the shapes of the following molecules. Also indicate whether they
are polar or nonpolar. The central atoms are shown in boldface.
HF
CO2
N2
H2S
Polar or nonpolar?
Polar or nonpolar?
Polar or nonpolar?
Polar or nonpolar?
Circle one
Circle one
Circle one
Circle one
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the Molecular Polarity Simulation to complete the following. Effect of Molecular Geometry on Polarity For each molecule: Sketch each molecule as shown in the simulation. Include arrows to show the bond dipoles as well as a molecular dipole (if present). Circle polar or nonpolar to indicate the polarity of the molecule. Name the molecular geometry (from Part A) H2O vs CO2 H2O CO2 polar nonpolar polar nonpolar Molecular Geometry Molecular Geometry Question 1a: How does the molecular geometry (linear vs bent) affect the molecular polarity?arrow_forwardWhether each molecule or polyatomic ion is polar or nonpolar. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. For example, if the molecule were HCI and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. molecule or polar or nonpolar? atom closest to polyatomic ion negative side O polar CH O nonpolar O polar NOCI O nonpolar O polar HI O nonpolar Explanation Check O 2021 Education. All Rig Reserved Terms of Use I Privacy Accessibility O V1 9:25 acer delete esc & %23 24 8. 9. 5 2 3 t е tab enter k f a Sarrow_forwardChemistry's quiz practice at homearrow_forward
- Decide whether each molecule or polyatomic ion is polar or nonpolar. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. molecule or polyatomic ion CC14 № 2 CO₂ polar or nonpolar? polar nonpolar polar nonpolar polar nonpolar X atom closest to negative side 0 Śarrow_forward1. Which of the following is a nonpolar molecule? Group of answer choices CCl4 NBr3 HCN H2O all molecules are polar 2. Which formula shown is incorrect for the name given? Group of answer choices ammonium cyanide: NH4CN strontium carbonate: SrCO3 potassium acetate: KC2H3O2 lithium sulfate: Li2SO4 calcium nitrate: CaNO3arrow_forwardWhich of the following molecules are nonpolar?arrow_forward
- Use the following information to determine the Lewis structure, find the electron and molecular geometry of the molecule, determine the angle of the molecule, and determine the polarity. If the atoms are not the same, you may assume that the difference in their electronegativities are between 0.4 and 2.0. Atom information: A: 6 valence electrons, CANNOT exceed the octet. Further from fluorine on the periodic table than X. X: 6 valence electrons, CANNOT exceed the octet. Closer to fluorine on the periodic table than A. Molecule: AX,2- Electron Geometry: [Select] Molecular Geometry: [Select ] Bond Angle: [ Select] Polarity: [Select]arrow_forwardWhich of the following molecules is nonpolar? Group of answer choices HCN SO2 PCl3 CBr4arrow_forwardBelow is the perspective drawings for BrF5. Below the structure are arrows indicating the overall direction of polarity or a “0” if the molecule is non polar.arrow_forward
- Answer the questions in the table below about the shape of the nitrosyl choride (NOC1) molecule. How many electron groups are around the central nitrogen atom? Note: one "electron group" means one lone pair, one single bond, one double bond, or one triple bond. What phrase best describes the arrangement of these electron groups around the central nitrogen atom? (You may need to use the scrollbar to see all the choices.) 0 (choose one) Xarrow_forwardor each of the following molecules: Use VSEPR theory to predict the molecular shape (linear, bent, trigonal planar, pyramidal or tetrahedral). State whether each molecule is overall polar or nonpolar. Molecule Molecular Shape Polar/Nonpolar CF4 BCl3 H2Sarrow_forwardWhich of the following is a polar molecule? Group of answer choices CH2O CF4 BH3 CO2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY