
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
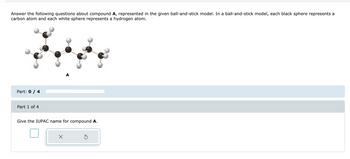
Transcribed Image Text:Answer the following questions about compound A, represented in the given ball-and-stick model. In a ball-and-stick model, each black sphere represents a
carbon atom and each white sphere represents a hydrogen atom.
Part: 0 / 4
Part 1 of 4
Give the IUPAC name for compound A.
☑
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Could you give the formula name and whether it is ionic or covalent? Please explain how to provide the correct formula name and why that is.arrow_forwardIdentify the names of the following molecules.arrow_forwardFor the following molecule, I need to identify several things, but I don't understand how to go about it. a) anomeric carbon b) carbon 1 c) carbon 5 d) which oxygen atoms in a hydroxyl group would point to the right in a Fischer Projectionarrow_forward
- Give the IUPAC name for each compound. Part 1 of 2 2-methylhept-3-ene n ☑ Part 2 of 2arrow_forwardA) what does the figure illustrate? B) Label the components in the figure pointed by the arrows and briefly mention the bonding partners involved in each casearrow_forwardAtoms A and B interact to form a compound, AB2. When measured, atom A has a partial negative charge and atom B has a partial positive charge. From this information, we can conclude what? Select only ONE answer choice. Note: - means "approximately equal to" , A > B means "A is greater than B" , and A B: AB2 is hydrophilic Not enough information to answer the questionarrow_forward
- What is the net charge on the following tripeptides (single-letter abbreviations). * YLV at pH 10.9 (the answer is -1.5 but I don't understand why)arrow_forwardClassify each of the following molecular formulas as an acyclic alkane or a cycloalkane. Part 1 of 4 C2H4: acyclic alkane ☑ Part 2 of 4 C23 H48 (Choose one) Part 3 of 4 C4H10 (Choose one) Part 4 of 4 C2652 (Choose one) Х ☑ ك ☑ ⑤arrow_forwardIn the following questions, consider the carbanion formed by the arrow pushing below: он | HC-C-R H ཋཙམཏིཏཡརིཡཐཱ པཱལཾ ཏིཡཏིཡ, B: H C-C-R Н Question 7 2 pts Below are a proposed resonance structure of the carbanion and rules of resonance. Which rules, if any, does the resonance structure break? (Select all that apply.) Ο Η | │ C-C-R H The formal charge of the molecule cannot change. ☐ Single (sigma) bonds cannot be broken. With the exception of carbocations, octet rules must be satisfied. ☐ All of the above rules are satisfied.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON