
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
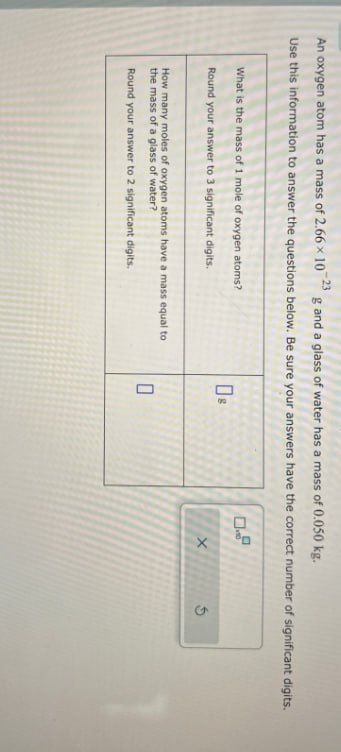
Transcribed Image Text:An oxygen atom has a mass of 2.66 × 10 23 g and a glass of water has a mass of 0.050 kg.
Use this information to answer the questions below. Be sure your answers have the correct number of significant digits.
What is the mass of 1 mole of oxygen atoms?
Round your answer to 3 significant digits.
How many moles of oxygen atoms have a mass equal to
the mass of a glass of water?
Round your answer to 2 significant digits.
☐ s
X
П
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- An unknown sample of a metal is 1.0 cm thick, 2.0 cm wide, and 10.0 cm long. Its mass is 54.0 g. Use data in Table 1.1 to identify the metal. (Remember that 1 cm3 = 1 mL.)arrow_forwardA person weighed 15 pennies on a balance and recorded the following masses: 3.112 g 3.109 g 3.059 g 2.467 g 3.079 g 2.518 g 3.129 g 2.545 g 3.050 g 3.053 g 3.054 g 3.072 g 3.081 g 3.131 g 3.064 g Curious about the results, he looked at the dates on each penny. Two of the light pennies were minted in 1983 and one in 1982. The dates on the 12 heavier pennies ranged from 1970 to 1982. Two of the 12 heavier pennies were minted in 1982. a. Do you think the Bureau of the Mint changed the way it made pennies? Explain. b. The person calculated the average mass of the 12 heavy pennies. He expressed this average as 3.0828 g 0.0482 g. What is wrong with the numbers in this result, and how should the value be expressed?arrow_forwardPlease solve the image.arrow_forward
- An iron atom has a mass of 9.27 x 10 - 23 g and a cooking pot has a mass of 0.500 kg. Use this information to answer the question below. Be sure your answers have the correct number of significant digits. What is the mass of 1 mole of iron atoms? Round your answer to 3 significant digits.arrow_forwardA raindrop has a mass of 50. mg and the Pacific Ocean has a mass of 7.08 x 10 kg. Use this information to answer the questions below. Be sure your answers have the correct number of significant digits. What is the mass of 1 mole of raindrops? X ? How many moles of raindrops are in the Pacific Ocean? 0arrow_forwardplease round to correct numbers of significant digitsarrow_forward
- A brick has a mass of 4.0 kg and the Earth has a mass of 6.0 × 10²7 Use this information to answer the questions below. Be sure your answers have the correct number of significant digits. What is the mass of 1 mole of bricks? How many moles of bricks have a mass equal to the mass of the Earth? 0 x10arrow_forwardhow do i solve this?arrow_forwardA brick has a mass of 4.0 kg and the Earth has a mass of 6.0 × 1027 g. Use this information to answer the question below. Be sure your answers have the correct number of significant digits. How many moles of bricks have a mass equal to the mass of the Earth? 0 X 3 ?arrow_forward
- Please do this work with base on the imagearrow_forwardAn iron atom has a mass of 9.27 × 10 2 -23 g and a cooking pot has a mass of 0.500 kg. Use this information to answer the question below. Be sure your answers have the correct number of significant digits. What is the mass of 1 mole of iron atoms? x10 garrow_forwardhow do i solvearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
