
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Draw the major resonance structures and include formal charge
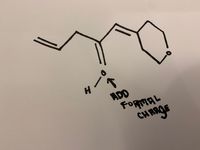
Transcribed Image Text:ADD
FORMAL
CH ARGE
Expert Solution
arrow_forward
Step 1
Conjugated double bonds (alternate double bonds and sigma bonds) are in resonance.
Isolated dienes donot show resonance.
Formula for calculation of formal charge on atom :-
Formal charge = valence electrons in neutral atom - 1/2(number of electrons in covalent bonds) - number of electrons in lone pairs
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Circle the most stable resonance structure.arrow_forwardTwo resonance structures are possible for the anion shown. One resonance form is given, but it is incomplete. Complete the given structure by adding nonbonding electrons and formal charges. Draw the remaining resonance structure, including nonbonding electrons and formal charges. Omit curved arrows. Structure A: complete the structure by adding Structure B: draw the remaining resonance structure, nonbonding electrons and formal charges. including nonbonding electrons and formal charges. Rings Erase Select Draw Rings More Erase Select Draw More H // C H. Harrow_forwardA) Consider the indicated molecule. Provide a resonance structure that has a +1 formal charge on both nitrogen atoms. H2N O2Narrow_forward
- Which of the following species is a valid resonance structure of A? Use curved arrows to show how A is converted to any valid resonance structure. When a compound is not a valid resonance structure of A, explain why not.arrow_forwardDraw all possible resonance structures for the species belowarrow_forwardMatch the atom with it's formal charge. C -H H ... H-C-P -c-H ai bi Prompts a Submitted Answers Choose a match Choose a match Choose a match Iarrow_forward
- For the given compound, draw all significant resonance forms and rank them from most significant to least significant. Briefly explain the rankings. Part 1 Let's begin by considering which resonance patterns are present. First, add curved arrow(s) to show the resonance using the following pattern: a pi bond between two atoms of differing electronegativity. Modify the second structure given to draw the new resonance structure. Include relevant formal charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds. H₂C CH₂ H₂C Edit Drawing CH₂ SUPPORTarrow_forwardDraw all 6 constitutional isomers of C3H6O each containing one double bond (one pibond). Draw each in Lewis structure and bondline format (zigzag structures )arrow_forwardA Lewis structure for the carbonate ion (CO-) is shown, but Add missing charges and non-bonding electrons. incomplete. Complete the structure by adding in formal charges and non-bonding electrons. Then draw the other two Select Draw Rings More Erase major resonance structures that fit the octet rule. Draw major resonance structure 2. Be sure to add Draw major resonance structure 3. Be sure to add charges and non-bonding electrons where appropriate. charges and non-bonding electrons where appropriate. Select Draw Rings Erase Select Draw Rings More More Erasearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY