
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
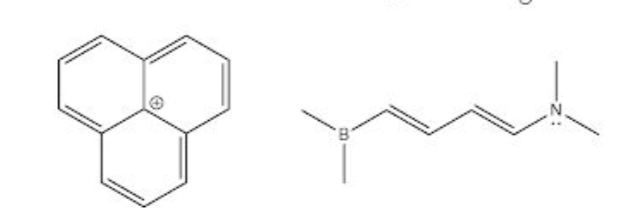
Could you draw all valid resonance structrues for these two? I understand the rules for drawing resonance structures but I miss a lot of them.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5. Draw several resonance contributors for the cyanate ion, [OCN]. Circle the two lowest energy contributors. In the actual anion, on which atom does the negative charge primarily reside? Justify your answer.arrow_forward33 Please draw the following Lewis structures (there are three). For each structure please identify the following: The total number of valence electrons (show work) A drawing of the correct Lewis structure The geometry of the molecule (required: give a one sentence justification of the geometry) The shape of the molecule (required: give a one sentence justification of the shape) Determine if the overall molecule is polar (required: give a one sentence justification of this answer) draw a Lewis structure and identify valence electrons, geometry, and shape. The three molecules are: AsI3the total number of valence electrons for AsI3: 2.GeSBr2 the geometry of GeSBr2: AsSe2– (notice the negative charge) the shape of AsSe2-:arrow_forwardHelp me pls ASAParrow_forward
- For the species below draw additional resonance structures (where all atoms have access to an octect of electrons) for species A, B, C, and D. Determine the bond order of the bond from the underlined C to underlined O for the lowest energy structure(s). You may need to evaluate the formal charge of the additional species you draw. These are species in an aprotic solvent, so only electrons may be moved, not hydrogen atoms. For each lettered structure below, input the bond order as an integer (1,2,etc) or improper fraction (4/3, 5/4, etc). A Structure Bond Order A HO: (f) H B B С Which structure A-D has the longest underlined C-O bond? :N=C=0: D Darrow_forward2) (cont.) In the box below each Lewis drawing, PRINT one of the three following ABBREVIATIONS to characterize that Lewis drawing's relevance to the molecule's ACTUAL structure. i) CRS (Contributing Resonance Structure) ii) RS (Resonance Structure but not a CRS) iii) NRS (Not a Resonance Structure)arrow_forward/Isl.exe/1o_u-IgNslkr7j8P3jH-liJQ0WQvqYPWviNXOF96XoVW3_W7J01F-2dL0GOOkIM8gnD. O REPRESENTATIONS OF ORGANIC MOLECULES Identifying skeletal resonance structures Decide whether each row is a set of resonance structures for a single molecule and select "yes" or "no" in the last column. structures resonance structures? O yes O no O yes O no O yes O no o yes no 74°F DELLarrow_forward
- 2. Tetrahydrocannabinolic acid (THCA), the precursor to THC, a psvchoactive: H.. • This molecule is missing eight lone pairs. Add them to the structure above • Check the formal charges of the oxygen atoms above. Do any of them have a non-zero formal charge? If so, add the charges to the appropriate oxygen atoms • THCA is a weak acid. Identify and circle the acidic hydrogen on the above structure • Draw a resonance structure of this molecule belowarrow_forwardOne, or both, of the following structural formulas may be incorrect (i. e., they do not represent a real compound) because they have atoms with an incorrect number of bonds. Which of the labeled atoms have the incorrect number of bonds? Specify the incorrect atoms by letter in alphabetical order without spaces or commas., i.e. bd. If there are no incorrect atoms, write none. a) b) H H lb lc d -C-0-H a H-N -C H bN H Harrow_forwardOne, or both, of the following structural formulas may be incorrect (i. e., they do not represent a real compound) because they have atoms with an incorrect number of bonds. Which of the labeled atoms have the incorrect number of bonds? Specify the incorrect atoms by letter in alphabetical order without spaces or commas., i.e. bd. If there are no incorrect atoms, write none. b c H-N=C-0=c-H a a) H H H od b) C-H Harrow_forward
- Which of the following species is a valid resonance structure of A? Use curved arrows to show how A is converted to any valid resonance structure. When a compound is not a valid resonance structure of A, explain why not.arrow_forwardit won't let me do OH togetherarrow_forward11:54 9 Question 48 of 53 Submit Draw the Lewis structure for thiosulfate (S203) with minimized formal changes. How many TOTAL likely resonance structures exist for S2032-? Hint: In this case, it is more stable (preferred) to place a negative charge on the larger atom. + Click to draw a new structure A) 1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY