
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:coo
CH,-č-CoA + H,0
Acetyl-CoA CoA
CH,
но-с-соо
coo
CH,
Citrate čoo
Oxaloacetate
NADH
Çoo
CH,
сн-соо
сн-он
NAD
CH,
Malate
но-с-н
Coo
Isocitrate CoO
NAD
coo
NADH + H
CH
+ co,
Fumarate
HC
Coo
CH,
CH,
a-ketoglutarate
FADH,
NAD
• COA
NADH + H
• co,
FAD
Succinate
čoo
CH,
-CH,
Succinyl-CoA
CH,
coo
GTP + COA
GDP• P.
COA
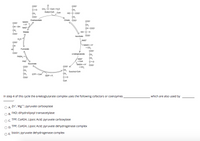
In step 4 of this cycle the a-ketoglutarate complex uses the following cofactors or coenzymes
which are also used by
O A. Zn", Mg**; pyruvate carboxylase
В.
FAD; dihydrolipoyl transacetylase
TPP, COASH, Lipoic Acid; pyruvate carboxylase
OP. TPP, COASH, Lipoic Acid; pyruvate dehydrogenase complex
O E. biotin; pyruvate dehydrogenase complex
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Please step by step answer please current answerarrow_forwardWhich of these statements about enzyme-catalyzed reactions is false? The activation energy for the catalyzed reaction is the same as for the uncatalyzed reaction, but the equilibrium constant is more favorable in the enzyme-catalyzed reaction. The Michaelis-Menten constant Km equals the [S] at which V = 1/2 V, max: At saturating levels of substrate, the rate of an enzyme-catalyzed reaction is proportional to the enzyme concentration. The rate of a reaction decreases steadily with time as substrate is depleted. If enough substrate is added, the normal V, of max a reaction can be attained even in the presence of a competitive inhibitor.arrow_forwardWhich one is the epimer of monosaccharide I?arrow_forward
- Do explain Question:- A common and effective treatment for arsenite poisoning involves administration of _____. aresenite chelator vitamin B1 a competitive sulfhydryl reagent NADPH a dihydrolipoyl inhibitorarrow_forwardThe five-digit code for all members of the family Enterobacteriaceae starts with the number 2 or 3. What does this indicate about their common biochemistry?arrow_forwardIs Oxaloacetate, a TCA cycle intermediate also a metabolite in gluconeogenesis? If not, which ones of these TCA cycle intermediates are also a metabolite in gluconeogenesis? Succinate Oxaloacetate Pyruvate Citratearrow_forward
- isomeease feuctose glucose Gal l. galactose giulose maltace maltose FRUctose maltose gałactose 2 glucose Answer these questions; make sure to justify your answers: 1. Do these yeast make isomerase? How can you tell? 2. Do these yeast make Gal1? How can you tell? 3. Do these yeast make maltase? how can you tell?arrow_forwardPeroxisomes will convert acetaldehyde using_______NAD+ into acetyl-COAarrow_forwardwhat macronutrient is used in Anaerobic Glycolysisarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON